YM 511Potent aromatase (CYP19) inhibitor CAS# 148869-05-0 |
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 148869-05-0 | SDF | Download SDF |
| PubChem ID | 177865 | Appearance | Powder |
| Formula | C16H12BrN5 | M.Wt | 354.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 25 mM in ethanol | ||
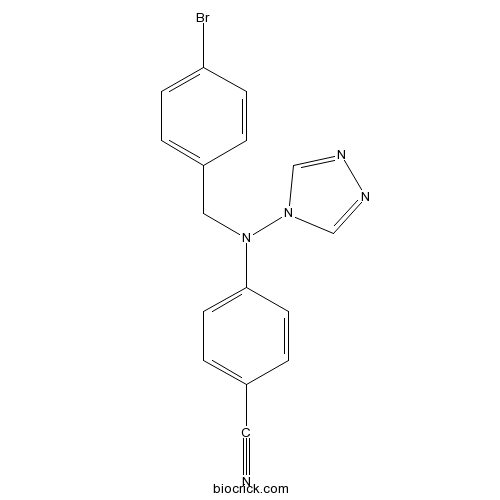
| Chemical Name | 4-[(4-bromophenyl)methyl-(1,2,4-triazol-4-yl)amino]benzonitrile | ||
| SMILES | C1=CC(=CC=C1CN(C2=CC=C(C=C2)C#N)N3C=NN=C3)Br | ||
| Standard InChIKey | GGPPBTSXFROGAE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12BrN5/c17-15-5-1-14(2-6-15)10-22(21-11-19-20-12-21)16-7-3-13(9-18)4-8-16/h1-8,11-12H,10H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Orally active, potent aromatase (CYP19) inhibitor (IC50 values are 0.4 and 0.12 nM at rat ovary and human placenta cells respectively) that only weakly inhibits the synthesis of other steroid hormones. Reduces plasma estrogen levels into ranges induced by ovariectomy and inhibits testosterone-induced breast cancer cell growth in vitro (IC50 = 0.13 nM). |

YM 511 Dilution Calculator

YM 511 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8233 mL | 14.1163 mL | 28.2326 mL | 56.4653 mL | 70.5816 mL |
| 5 mM | 0.5647 mL | 2.8233 mL | 5.6465 mL | 11.2931 mL | 14.1163 mL |
| 10 mM | 0.2823 mL | 1.4116 mL | 2.8233 mL | 5.6465 mL | 7.0582 mL |
| 50 mM | 0.0565 mL | 0.2823 mL | 0.5647 mL | 1.1293 mL | 1.4116 mL |
| 100 mM | 0.0282 mL | 0.1412 mL | 0.2823 mL | 0.5647 mL | 0.7058 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ivabradine HCl
Catalog No.:BCC4350
CAS No.:148849-67-6
- Rutamarin
Catalog No.:BCN7509
CAS No.:14882-94-1
- Bismuth Subsalicylate
Catalog No.:BCC3739
CAS No.:14882-18-9
- Carboxy-PTIO, potassium salt
Catalog No.:BCC6789
CAS No.:148819-94-7
- (R)-2-Methylcysteine HCl
Catalog No.:BCC4017
CAS No.:148766-37-4
- Tyrphostin AG 879
Catalog No.:BCC4514
CAS No.:148741-30-4
- SB 204070
Catalog No.:BCC5752
CAS No.:148688-01-1
- L-733,060 hydrochloride
Catalog No.:BCC5707
CAS No.:148687-76-7
- GR 127935 hydrochloride
Catalog No.:BCC7081
CAS No.:148642-42-6
- Fmoc-Prolinol
Catalog No.:BCC2710
CAS No.:148625-77-8
- 3-O-Methylquercetin
Catalog No.:BCN1660
CAS No.:1486-70-0
- 3-O-Methylquercetin tetraacetate
Catalog No.:BCN1659
CAS No.:1486-69-7
- HATU
Catalog No.:BCC2813
CAS No.:148893-10-1
- Fmoc-L-Arg(Aloc)2-OH
Catalog No.:BCC2564
CAS No.:148893-34-9
- 3-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl-2-propenal
Catalog No.:BCC8600
CAS No.:148901-68-2
- Fmoc-Inp-OH
Catalog No.:BCC3266
CAS No.:148928-15-8
- Erythritol
Catalog No.:BCN1664
CAS No.:149-32-6
- Scopolamine butylbromide
Catalog No.:BCN5006
CAS No.:149-64-4
- Gallic acid
Catalog No.:BCN1668
CAS No.:149-91-7
- D-Isomenthol
Catalog No.:BCN8540
CAS No.:23283-97-8
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- (S)-WAY 100135 dihydrochloride
Catalog No.:BCC6993
CAS No.:149007-54-5
- H-D-Trp-OMe.HCl
Catalog No.:BCC3118
CAS No.:14907-27-8
- Brusatol
Catalog No.:BCN8278
CAS No.:14907-98-3
Studies on aromatase inhibitors. I. Synthesis and biological evaluation of 4-amino-4H-1,2,4-triazole derivatives.[Pubmed:8904814]
Chem Pharm Bull (Tokyo). 1996 Oct;44(10):1871-9.
Various 4-N-substituted amino-4H-1,2,4-triazole derivatives were synthesized and evaluated for aromatase-inhibitory activity (in vitro) and for pregnant mare serum gonadotropin (PMSG)-induced estrogen synthesis-inhibitory activity (in vivo). The 4-(4-cyanophenyl) amino derivative and 4-(4-nitrophenyl)amino derivative, each possessing a strong electron-withdrawing group on the phenyl moiety, showed potent aromatase-inhibitory activity. Structure-activity relationship studies indicated that 4-[(4-bromobenzyl)(4-cyanophenyl)amino]-4H-1,2,4-triazole (5k, YM511) is a highly potent aromatase inhibitor with IC50 values of 0.4 and 0.12 nM in in vitro experiments using rat ovary and human placenta, respectively, and an in vivo ED50 of 0.002 mg/kg in rats on oral administration. YM511 was also a weak inhibitor of other steroid hormone synthesis enzymes. These data suggest that YM511 is a highly selective aromatase inhibitor and may be a useful agent for the treatment of estrogen-dependent diseases such as breast cancer.


