ErythritolCAS# 149-32-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 149-32-6 | SDF | Download SDF |
| PubChem ID | 8998 | Appearance | Oil |
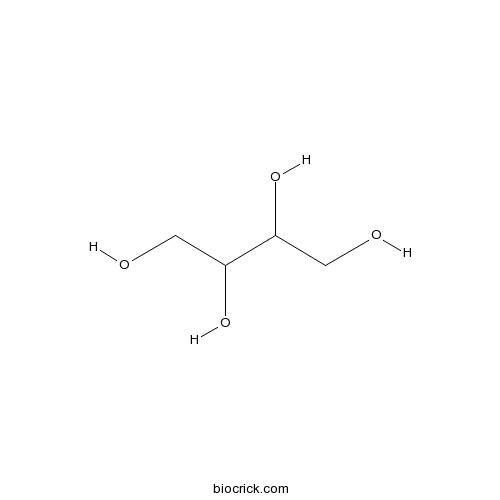
| Formula | C4H10O4 | M.Wt | 122.1 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | >6mg/mL in DMSO | ||
| Chemical Name | butane-1,2,3,4-tetrol | ||
| SMILES | C(C(C(CO)O)O)O | ||
| Standard InChIKey | UNXHWFMMPAWVPI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H10O4/c5-1-3(7)4(8)2-6/h3-8H,1-2H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Erythritol is a biological sweetener with applications in food and pharmaceutical industries, it is also used as a functional sugar substitute in special foods for people with diabetes and obesity because of its unique nutritional properties. Erythritol consumption acutely improved small vessel endothelial function, and chronic treatment reduced central aortic stiffness, may be as a preferred sugar substitute for patients with diabetes mellitus. |
| In vitro | Biotechnological production of erythritol and its applications.[Pubmed: 20186409 ]Appl Microbiol Biotechnol. 2010 Apr;86(4):1017-25.Erythritol, a four-carbon polyol, is a biological sweetener with applications in food and pharmaceutical industries. It is also used as a functional sugar substitute in special foods for people with diabetes and obesity because of its unique nutritional properties. |
| In vivo | Effects of erythritol on endothelial function in patients with type 2 diabetes mellitus: a pilot study.[Pubmed: 24366423]Acta Diabetol. 2014;51(3):513-6.Sugar substitutes are important in the dietary management of diabetes mellitus. Erythritol is a non-caloric dietary bulk sweetener that reverses endothelial dysfunction in diabetic rats. |
| Kinase Assay | Erythritol feeds the pentose phosphate pathway via three new isomerases leading to D-erythrose-4-phosphate in Brucella.[Pubmed: 25453104]Proc Natl Acad Sci U S A. 2014 Dec 16;111(50):17815-20.Erythritol is an important nutrient for several α-2 Proteobacteria, including N2-fixing plant endosymbionts and Brucella, a worldwide pathogen that finds this four-carbon polyol in genital tissues. |
| Structure Identification | Int J Pharm. 2013 Oct 15;455(1-2):132-7.A completely solvent-free process for the improvement of erythritol compactibility.[Pubmed: 23891654]We obtained improvement of Erythritol compactibility by formulating composite particles composed of Erythritol and porous silica using a twin-screw kneader.
|

Erythritol Dilution Calculator

Erythritol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.19 mL | 40.95 mL | 81.9001 mL | 163.8002 mL | 204.7502 mL |
| 5 mM | 1.638 mL | 8.19 mL | 16.38 mL | 32.76 mL | 40.95 mL |
| 10 mM | 0.819 mL | 4.095 mL | 8.19 mL | 16.38 mL | 20.475 mL |
| 50 mM | 0.1638 mL | 0.819 mL | 1.638 mL | 3.276 mL | 4.095 mL |
| 100 mM | 0.0819 mL | 0.4095 mL | 0.819 mL | 1.638 mL | 2.0475 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fmoc-Inp-OH
Catalog No.:BCC3266
CAS No.:148928-15-8
- 3-[2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl-2-propenal
Catalog No.:BCC8600
CAS No.:148901-68-2
- Fmoc-L-Arg(Aloc)2-OH
Catalog No.:BCC2564
CAS No.:148893-34-9
- HATU
Catalog No.:BCC2813
CAS No.:148893-10-1
- YM 511
Catalog No.:BCC6002
CAS No.:148869-05-0
- Ivabradine HCl
Catalog No.:BCC4350
CAS No.:148849-67-6
- Rutamarin
Catalog No.:BCN7509
CAS No.:14882-94-1
- Bismuth Subsalicylate
Catalog No.:BCC3739
CAS No.:14882-18-9
- Carboxy-PTIO, potassium salt
Catalog No.:BCC6789
CAS No.:148819-94-7
- (R)-2-Methylcysteine HCl
Catalog No.:BCC4017
CAS No.:148766-37-4
- Tyrphostin AG 879
Catalog No.:BCC4514
CAS No.:148741-30-4
- SB 204070
Catalog No.:BCC5752
CAS No.:148688-01-1
- Scopolamine butylbromide
Catalog No.:BCN5006
CAS No.:149-64-4
- Gallic acid
Catalog No.:BCN1668
CAS No.:149-91-7
- D-Isomenthol
Catalog No.:BCN8540
CAS No.:23283-97-8
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- (S)-WAY 100135 dihydrochloride
Catalog No.:BCC6993
CAS No.:149007-54-5
- H-D-Trp-OMe.HCl
Catalog No.:BCC3118
CAS No.:14907-27-8
- Brusatol
Catalog No.:BCN8278
CAS No.:14907-98-3
- AG 825
Catalog No.:BCC7113
CAS No.:149092-50-2
- Brugine
Catalog No.:BCN1899
CAS No.:14912-30-2
- Cratoxylone
Catalog No.:BCN3875
CAS No.:149155-01-1
- Homaloside D
Catalog No.:BCN1661
CAS No.:149155-19-1
- Eriodictyol chalcone
Catalog No.:BCN8276
CAS No.:14917-41-0
A completely solvent-free process for the improvement of erythritol compactibility.[Pubmed:23891654]
Int J Pharm. 2013 Oct 15;455(1-2):132-7.
OBJECTIVE: We obtained improvement of Erythritol compactibility by formulating composite particles composed of Erythritol and porous silica using a twin-screw kneader. METHODS: Erythritol-based tablets formulated with composite particles were directly compacted, and we estimated their hardness and the friability. The compression properties of the Erythritol powder bed including composite particles were estimated using a Heckel analysis and force-displacement profiles, and we investigated the physical states of the composite particles by powder X-ray diffractometry, a thermal analysis and a nitrogen gas adsorption study. RESULTS: A direct-compacted Erythritol tablet formulated with composite particles, prepared at the melting temperature of Erythritol (120 degrees C), exhibited high hardness and low friability. A pressure transmission study revealed the higher plasticity and lower elasticity of an Erythritol powder bed formulated with composite particles prepared at 120 degrees C. Physical states of the composite particles indicated that Erythritol in the composite particles was adsorbed onto porous silica with a subsequent decrease in the Erythritol crystallinity as a result of high mechanical force at the melting temperature of Erythritol. CONCLUSION: The improvement of the Erythritol compactibility formulated with composite particles through processing with a twin-screw kneader at 120 degrees C. This was affected by the reduction of the Erythritol crystallinity in the composite particles.
Biotechnological production of erythritol and its applications.[Pubmed:20186409]
Appl Microbiol Biotechnol. 2010 Apr;86(4):1017-25.
Erythritol, a four-carbon polyol, is a biological sweetener with applications in food and pharmaceutical industries. It is also used as a functional sugar substitute in special foods for people with diabetes and obesity because of its unique nutritional properties. Erythritol is produced by microbial methods using mostly osmophilic yeasts and has been produced commercially using mutant strains of Aureobasidium sp. and Pseudozyma tsukubaensis. Due to the high yield and productivity in the industrial scale of production, Erythritol serves as an inexpensive starting material for the production of other sugars. This review focuses on the approaches for the efficient Erythritol production, strategies used to enhance Erythritol productivity in microbes, and the potential biotechnological applications of Erythritol.
Erythritol feeds the pentose phosphate pathway via three new isomerases leading to D-erythrose-4-phosphate in Brucella.[Pubmed:25453104]
Proc Natl Acad Sci U S A. 2014 Dec 16;111(50):17815-20.
Erythritol is an important nutrient for several alpha-2 Proteobacteria, including N2-fixing plant endosymbionts and Brucella, a worldwide pathogen that finds this four-carbon polyol in genital tissues. Erythritol metabolism involves phosphorylation to L-Erythritol-4-phosphate by the kinase EryA and oxidation of the latter to L-3-tetrulose 4-phosphate by the dehydrogenase EryB. It is accepted that further steps involve oxidation by the putative dehydrogenase EryC and subsequent decarboxylation to yield triose-phosphates. Accordingly, growth on Erythritol as the sole C source should require aldolase and fructose-1,6-bisphosphatase to produce essential hexose-6-monophosphate. However, we observed that a mutant devoid of fructose-1,6-bisphosphatases grew normally on Erythritol and that EryC, which was assumed to be a dehydrogenase, actually belongs to the xylose isomerase superfamily. Moreover, we found that TpiA2 and RpiB, distant homologs of triose phosphate isomerase and ribose 5-phosphate isomerase B, were necessary, as previously shown for Rhizobium. By using purified recombinant enzymes, we demonstrated that L-3-tetrulose-4-phosphate was converted to D-erythrose 4-phosphate through three previously unknown isomerization reactions catalyzed by EryC (tetrulose-4-phosphate racemase), TpiA2 (D-3-tetrulose-4-phosphate isomerase; renamed EryH), and RpiB (D-erythrose-4-phosphate isomerase; renamed EryI), a pathway fully consistent with the isotopomer distribution of the erythrose-4-phosphate-derived amino acids phenylalanine and tyrosine obtained from bacteria grown on (13)C-labeled Erythritol. D-erythrose-4-phosphate is then converted by enzymes of the pentose phosphate pathway to glyceraldehyde 3-phosphate and fructose 6-phosphate, thus bypassing fructose-1,6-bisphosphatase. This is the first description to our knowledge of a route feeding carbohydrate metabolism exclusively via D-erythrose 4-phosphate, a pathway that may provide clues to the preferential metabolism of Erythritol by Brucella and its role in pathogenicity.
Effects of erythritol on endothelial function in patients with type 2 diabetes mellitus: a pilot study.[Pubmed:24366423]
Acta Diabetol. 2014;51(3):513-6.
Sugar substitutes are important in the dietary management of diabetes mellitus. Erythritol is a non-caloric dietary bulk sweetener that reverses endothelial dysfunction in diabetic rats. We completed a pilot study to examine the effects of Erythritol on vascular function in patients with type 2 diabetes mellitus. Participants (n = 24) consumed Erythritol 36 g/day as an orange-flavored beverage for 4 weeks and a single dose of 24 g during the baseline and final visits. We assessed vascular function before and after acute (2 h) and chronic (4 weeks) Erythritol consumption. Acute Erythritol improved endothelial function measured by fingertip peripheral arterial tonometry (0.52 +/- 0.48 to 0.87 +/- 0.29 au, P = 0.005). Chronic Erythritol decreased central pulse pressure (47 +/- 13 to 41 +/- 9 mmHg, P = 0.02) and tended to decrease carotid-femoral pulse wave velocity (P = 0.06). Thus, Erythritol consumption acutely improved small vessel endothelial function, and chronic treatment reduced central aortic stiffness. Erythritol may be a preferred sugar substitute for patients with diabetes mellitus.


