Ivacaftor hydrateCFTR Potentiator CAS# 1134822-07-3 |
- BMS-509744
Catalog No.:BCC1424
CAS No.:439575-02-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1134822-07-3 | SDF | Download SDF |
| PubChem ID | 78357769 | Appearance | Powder |
| Formula | C24H30N2O4 | M.Wt | 410.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | VX-770 hydrate | ||
| Solubility | 25℃: DMSO | ||
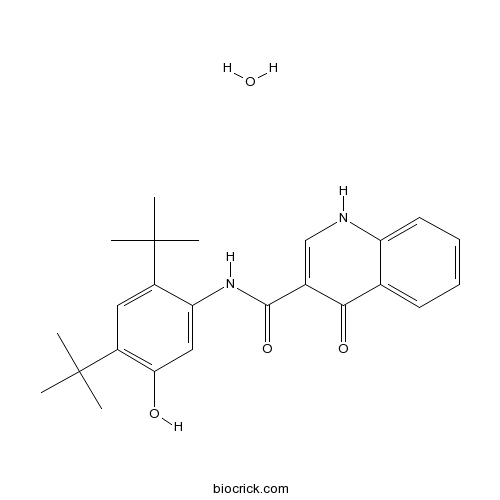
| Chemical Name | N-(2,4-ditert-butyl-5-hydroxyphenyl)-4-oxo-1H-quinoline-3-carboxamide;hydrate | ||
| SMILES | CC(C)(C)C1=CC(=C(C=C1NC(=O)C2=CNC3=CC=CC=C3C2=O)O)C(C)(C)C.O | ||
| Standard InChIKey | MYELKYHBCRDZNH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H28N2O3.H2O/c1-23(2,3)16-11-17(24(4,5)6)20(27)12-19(16)26-22(29)15-13-25-18-10-8-7-9-14(18)21(15)28;/h7-13,27H,1-6H3,(H,25,28)(H,26,29);1H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ivacaftor hydrate is an orally bioavailable CFTR potentiator, used for cystic fibrosis treatment.In Vitro:Ivacaftor (10 µM) increases the PC secretion activity by 3-fold for ABCB4-G535D, 13.7-fold for ABCB4-G536R, 6.7-fold for ABCB4-S1076C, 9.4-fold for ABCB4-S1176L, and 5.7-fold for ABCB4-G1178S. Ivacaftor corrects the functional defect of ABCB4 mutants[1]. Ivacaftor (10 μM) significantly increases CFTR activity in W1282X-expressing cells compared to R1162X CFTR cells[2]. Ivacaftor shows no significant activity against 160 targets tested including the GABAA benzodiazepine receptor. Ivacaftor increases the chloride secretion with an EC50 of 0.236 ± 0.200 μM, a 10-fold shift in potency compared to the F508del HBEs[3]. In recombinant cells, VX-770 increases CFTR channel open probability (Po) in both the F508del processing mutation and the G551D gating mutation. VX-770 increases forskolin-stimulated IT in temperature-corrected F508del-FRT cells by appr 6-fold with an EC50 of 25 nM[4].In Vivo:Ivacaftor (1-200 mg/kg, p.o.) exhibits good oral bioavailability in rat[3]. References: | |||||

Ivacaftor hydrate Dilution Calculator

Ivacaftor hydrate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.436 mL | 12.18 mL | 24.3599 mL | 48.7199 mL | 60.8999 mL |
| 5 mM | 0.4872 mL | 2.436 mL | 4.872 mL | 9.744 mL | 12.18 mL |
| 10 mM | 0.2436 mL | 1.218 mL | 2.436 mL | 4.872 mL | 6.09 mL |
| 50 mM | 0.0487 mL | 0.2436 mL | 0.4872 mL | 0.9744 mL | 1.218 mL |
| 100 mM | 0.0244 mL | 0.1218 mL | 0.2436 mL | 0.4872 mL | 0.609 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 25 nM (F508del-CFTR);100 nM (G551D-CFTR) [1] Ivacaftor (VX-770) is a CFTR Potentiator approved for patients with the G551D mutation of cystic fibrosis, which accounts for 4-5% cases of cystic fibrosis. in vitro: In recombinant cells VX-770 increased CFTR channel open probability (P(o)) in both the F508del processing mutation and the G551D gating mutation. VX-770 also increased Cl(-) secretion in cultured human CF bronchial epithelia (HBE) carrying the G551D gating mutation on one allele and the F508del processing mutation on the other allele by approximately 10-fold, to approximately 50% of that observed in HBE isolated from individuals without CF [1]. in vivo: At day 28, in the group of subjects who received 150 mg of VX-770, the median change in the nasal potential difference (in response to the administration of a chloride-free isoproterenol solution) from baseline was -3.5 mV (range, -8.3 to 0.5; P=0.02 for the within-subject comparison, P=0.13 vs. placebo), and the median change in the level of sweat chloride was -59.5 mmol per liter (range, -66.0 to -19.0; P=0.008 within-subject, P=0.02 vs. placebo) [2]. Toxicity: Six severe adverse events occurred in two subjects (diffuse macular rash in one subject and five incidents of elevated blood and urine glucose levels in one subject with diabetes). All severe adverse events resolved without the discontinuation of VX-770 [2]. Clinical trial: Roll-Over Study of Ivacaftor in Cystic Fibrosis Pediatric Subjects With a CFTR Gating Mutation. Phase 3
- Epidanshenspiroketallactone
Catalog No.:BCN3142
CAS No.:113472-19-8
- Rhein-8-glucoside calcium salt
Catalog No.:BCN6349
CAS No.:113443-70-2
- VER 155008
Catalog No.:BCC2338
CAS No.:1134156-31-2
- Jatamanvaltrate B
Catalog No.:BCN7128
CAS No.:1134138-66-1
- 2-Aminophenyl phenyl sulfide
Catalog No.:BCC8553
CAS No.:1134-94-7
- Baclofen
Catalog No.:BCC8839
CAS No.:1134-47-0
- 2-Amino-4-phenylphenol
Catalog No.:BCC8534
CAS No.:1134-36-7
- γ-Truxilline
Catalog No.:BCN1948
CAS No.:113350-56-4
- neo-Truxilline
Catalog No.:BCN1949
CAS No.:113350-54-2
- GDC-0834
Catalog No.:BCC5115
CAS No.:1133432-50-4
- ABT-333
Catalog No.:BCC4129
CAS No.:1132935-63-7
- (3S)-Hydrangenol 8-O-glucoside pentaacetate
Catalog No.:BCN1616
CAS No.:113270-99-8
- Ivacaftor benzenesulfonate
Catalog No.:BCC1663
CAS No.:1134822-09-5
- Ferulic acid
Catalog No.:BCN5948
CAS No.:1135-24-6
- Moxidectin
Catalog No.:BCC5309
CAS No.:113507-06-5
- Tracazolate hydrochloride
Catalog No.:BCC7115
CAS No.:1135210-68-2
- VU 0357017 hydrochloride
Catalog No.:BCC7907
CAS No.:1135242-13-5
- VU 0255035
Catalog No.:BCC7766
CAS No.:1135243-19-4
- SR 59230A hydrochloride
Catalog No.:BCC7094
CAS No.:1135278-41-9
- CGP 78608 hydrochloride
Catalog No.:BCC7087
CAS No.:1135278-54-4
- 3'-Fluorobenzylspiperone maleate
Catalog No.:BCC6752
CAS No.:1135278-61-3
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- Altanserin hydrochloride
Catalog No.:BCC7183
CAS No.:1135280-78-2
- 6-Bnz-cAMP sodium salt
Catalog No.:BCC8043
CAS No.:1135306-29-4
Therapeutic options for hydrating airway mucus in cystic fibrosis.[Pubmed:25823699]
Pharmacology. 2015;95(3-4):117-32.
BACKGROUND: In cystic fibrosis (CF), genetic mutations in the CF transmembrane conductance regulator (CFTR) gene cause reduced chloride efflux from ciliated airway epithelial cells. This results in a reduction in periciliary liquid (PCL) depth of the airway surface liquid due to associated reduced water efflux. PCL layer dehydration reduces mucociliary clearance (MCC), leading to airway obstruction (reduced airflow and inflammation due to pathogen invasion) with mucus plug formation. SUMMARY: Rehydrating mucus increases MCC. Mucus hydration can be achieved by direct hydration (administering osmotic agents to set up an osmotic gradient), using CFTR modulators to correct dysfunctional CFTR, or it can be achieved pharmacologically (targeting other ion channels on airway epithelial cells). Key Messages: The molecular mechanisms of several therapies are discussed in the context of pre-clinical and clinical trial studies. Currently, only the osmotic agent 7% hypertonic saline and the CFTR 'potentiator' VX-770 (ivacaftor) are used clinically to hydrate mucus. Emerging therapies include the osmotic agent mannitol (Bronchitol), the intracellular Ca(2+)-raising agent Moli1901/lancovutide, the CFTR potentiator sildenafil [phosphodiesterase type 5 (PDE5) inhibitor] and the CFTR 'corrector' VX-809 (lumacaftor). Other CFTR correctors (e.g. 'chemical chaperones') are also showing pre-clinical promise.


