GDC-0834CAS# 1133432-50-4 |
- AZD6244 (Selumetinib)
Catalog No.:BCC3624
CAS No.:606143-52-6
- Trametinib (GSK1120212)
Catalog No.:BCC1282
CAS No.:871700-17-3
- Vemurafenib (PLX4032, RG7204)
Catalog No.:BCC1269
CAS No.:918504-65-1
- PLX-4720
Catalog No.:BCC1280
CAS No.:918505-84-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1133432-50-4 | SDF | Download SDF |
| PubChem ID | 25234917 | Appearance | Powder |
| Formula | C33H36N6O3S | M.Wt | 596.74 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (167.58 mM; Need ultrasonic) | ||
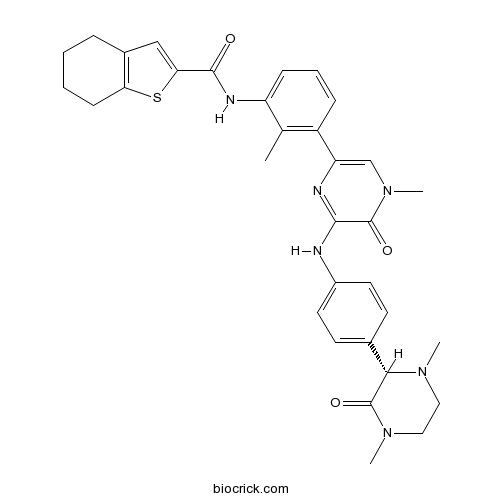
| Chemical Name | N-[3-[6-[4-[(2S)-1,4-dimethyl-3-oxopiperazin-2-yl]anilino]-4-methyl-5-oxopyrazin-2-yl]-2-methylphenyl]-4,5,6,7-tetrahydro-1-benzothiophene-2-carboxamide | ||
| SMILES | CC1=C(C=CC=C1NC(=O)C2=CC3=C(S2)CCCC3)C4=CN(C(=O)C(=N4)NC5=CC=C(C=C5)C6C(=O)N(CCN6C)C)C | ||
| Standard InChIKey | CDOOFZZILLRUQH-LJAQVGFWSA-N | ||
| Standard InChI | InChI=1S/C33H36N6O3S/c1-20-24(9-7-10-25(20)36-31(40)28-18-22-8-5-6-11-27(22)43-28)26-19-39(4)33(42)30(35-26)34-23-14-12-21(13-15-23)29-32(41)38(3)17-16-37(29)2/h7,9-10,12-15,18-19,29H,5-6,8,11,16-17H2,1-4H3,(H,34,35)(H,36,40)/t29-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GDC-0834 (S-enantiomer) is the S-enantiomer of GDC-0834. GDC-0834 is a potent and selective BTK inhibitor. References: | |||||

GDC-0834 Dilution Calculator

GDC-0834 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6758 mL | 8.3789 mL | 16.7577 mL | 33.5154 mL | 41.8943 mL |
| 5 mM | 0.3352 mL | 1.6758 mL | 3.3515 mL | 6.7031 mL | 8.3789 mL |
| 10 mM | 0.1676 mL | 0.8379 mL | 1.6758 mL | 3.3515 mL | 4.1894 mL |
| 50 mM | 0.0335 mL | 0.1676 mL | 0.3352 mL | 0.6703 mL | 0.8379 mL |
| 100 mM | 0.0168 mL | 0.0838 mL | 0.1676 mL | 0.3352 mL | 0.4189 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GDC-0834 is a novel potent and selective BTK inhibitor with IC50 of 5.9 nM.
- ABT-333
Catalog No.:BCC4129
CAS No.:1132935-63-7
- (3S)-Hydrangenol 8-O-glucoside pentaacetate
Catalog No.:BCN1616
CAS No.:113270-99-8
- (3R)-Hydrangenol 8-O-glucoside pentaacetate
Catalog No.:BCN1617
CAS No.:113270-98-7
- H-Phe(4-F)-OH
Catalog No.:BCC3216
CAS No.:1132-68-9
- PPPA
Catalog No.:BCC7309
CAS No.:113190-92-4
- Linopirdine dihydrochloride
Catalog No.:BCC7231
CAS No.:113168-57-3
- nor-Binaltorphimine dihydrochloride
Catalog No.:BCC6614
CAS No.:113158-34-2
- Physalin L
Catalog No.:BCN2312
CAS No.:113146-74-0
- (S)-(+)-Niguldipine hydrochloride
Catalog No.:BCC6947
CAS No.:113145-69-0
- 3-Deoxysappanone B
Catalog No.:BCN6012
CAS No.:113122-54-6
- 7-O-Methylrosmanol
Catalog No.:BCN7276
CAS No.:113085-62-4
- VU 0155069
Catalog No.:BCC7715
CAS No.:1130067-06-9
- neo-Truxilline
Catalog No.:BCN1949
CAS No.:113350-54-2
- γ-Truxilline
Catalog No.:BCN1948
CAS No.:113350-56-4
- 2-Amino-4-phenylphenol
Catalog No.:BCC8534
CAS No.:1134-36-7
- Baclofen
Catalog No.:BCC8839
CAS No.:1134-47-0
- 2-Aminophenyl phenyl sulfide
Catalog No.:BCC8553
CAS No.:1134-94-7
- Jatamanvaltrate B
Catalog No.:BCN7128
CAS No.:1134138-66-1
- VER 155008
Catalog No.:BCC2338
CAS No.:1134156-31-2
- Rhein-8-glucoside calcium salt
Catalog No.:BCN6349
CAS No.:113443-70-2
- Epidanshenspiroketallactone
Catalog No.:BCN3142
CAS No.:113472-19-8
- Ivacaftor hydrate
Catalog No.:BCC1664
CAS No.:1134822-07-3
- Ivacaftor benzenesulfonate
Catalog No.:BCC1663
CAS No.:1134822-09-5
- Ferulic acid
Catalog No.:BCN5948
CAS No.:1135-24-6
A novel reaction mediated by human aldehyde oxidase: amide hydrolysis of GDC-0834.[Pubmed:25845827]
Drug Metab Dispos. 2015 Jun;43(6):908-15.
GDC-0834, a Bruton's tyrosine kinase inhibitor investigated as a potential treatment of rheumatoid arthritis, was previously reported to be extensively metabolized by amide hydrolysis such that no measurable levels of this compound were detected in human circulation after oral administration. In vitro studies in human liver cytosol determined that GDC-0834 (R)-N-(3-(6-(4-(1,4-dimethyl-3-oxopiperazin-2-yl)phenylamino)-4-methyl-5-oxo- 4,5-dihydropyrazin-2-yl)-2-methylphenyl)-4,5,6,7-tetrahydrobenzo[b] thiophene-2-carboxamide) was rapidly hydrolyzed with a CLint of 0.511 ml/min per milligram of protein. Aldehyde oxidase (AO) and carboxylesterase (CES) were putatively identified as the enzymes responsible after cytosolic fractionation and mass spectrometry-proteomics analysis of the enzymatically active fractions. Results were confirmed by a series of kinetic experiments with inhibitors of AO, CES, and xanthine oxidase (XO), which implicated AO and CES, but not XO, as mediating GDC-0834 amide hydrolysis. Further supporting the interaction between GDC-0834 and AO, GDC-0834 was shown to be a potent reversible inhibitor of six known AO substrates with IC50 values ranging from 0.86 to 1.87 muM. Additionally, in silico modeling studies suggest that GDC-0834 is capable of binding in the active site of AO with the amide bond of GDC-0834 near the molybdenum cofactor (MoCo), orientated in such a way to enable potential nucleophilic attack on the carbonyl of the amide bond by the hydroxyl of MoCo. Together, the in vitro and in silico results suggest the involvement of AO in the amide hydrolysis of GDC-0834.
Validation and application of a liquid chromatography-tandem mass spectrometric method for the determination of GDC-0834 and its metabolite in human plasma using semi-automated 96-well protein precipitation.[Pubmed:22311651]
Biomed Chromatogr. 2012 Nov;26(11):1444-51.
A liquid chromatographic-tandem mass spectrometric (LC-MS/MS) method was developed and validated for the determination of GDC-0834 and its amide hydrolysis metabolite (M1) in human plasma to support clinical development. The method consisted of semi-automated 96-well protein precipitation extraction for sample preparation and LC-MS/MS analysis in positive ion mode using TurboIonSpray(R) for analysis. D6-GDC-0834 and D6-M1 metabolite were used as internal standards. A linear regression (weighted 1/concentration(2) ) was used to fit calibration curves over the concentration range of 1 - 500 ng/mL for both GDC-0834 and M1 metabolite. The accuracy (percentage bias) at the lower limit of quantitation (LLOQ) was 5.20 and 0.100% for GDC-0834 and M1 metabolite, respectively. The precision (CV) for samples at the LLOQ was 3.13-8.84 and 5.20-8.93% for GDC-0834 and M1 metabolite, respectively. For quality control samples at 3, 200 and 400 ng/mL, the between-run CV was GDC-0834 and GDC-0834 and from -6.73 to 2.21% for M1 metabolite. GDC-0834 and M1 metabolite were stable in human plasma for 31 days at -20 and -70 degrees C. This method was successfully applied to support a GDC-0834 human pharmacokinetic-based study.
Potent and selective Bruton's tyrosine kinase inhibitors: discovery of GDC-0834.[Pubmed:25701252]
Bioorg Med Chem Lett. 2015 Mar 15;25(6):1333-7.
SAR studies focused on improving the pharmacokinetic (PK) properties of the previously reported potent and selective Btk inhibitor CGI-1746 (1) resulted in the clinical candidate GDC-0834 (2), which retained the potency and selectivity of CGI-1746, but with much improved PK in preclinical animal models. Structure based design efforts drove this work as modifications to 1 were investigated at both the solvent exposed region as well as 'H3 binding pocket'. However, in vitro metabolic evaluation of 2 revealed a non CYP-mediated metabolic process that was more prevalent in human than preclinical species (mouse, rat, dog, cyno), leading to a high-level of uncertainly in predicting human pharmacokinetics. Due to its promising potency, selectivity, and preclinical efficacy, a single dose IND was filed and 2 was taken in to a single dose phase I trial in healthy volunteers to quickly evaluate the human pharmacokinetics. In human, 2 was found to be highly labile at the exo-cyclic amide bond that links the tetrahydrobenzothiophene moiety to the central aniline ring, resulting in insufficient parent drug exposure. This information informed the back-up program and discovery of improved inhibitors.
Significant species difference in amide hydrolysis of GDC-0834, a novel potent and selective Bruton's tyrosine kinase inhibitor.[Pubmed:21742900]
Drug Metab Dispos. 2011 Oct;39(10):1840-9.
(R)-N-(3-(6-(4-(1,4-dimethyl-3-oxopiperazin-2-yl)phenylamino)-4-methyl-5-oxo-4,5- dihydropyrazin-2-yl)-2-methylphenyl)-4,5,6,7-tetrahydrobenzo[b]thiophene-2-carbox amide (GDC-0834) is a potent and selective inhibitor of Bruton's tyrosine kinase (BTK), investigated as a potential treatment for rheumatoid arthritis. In vitro metabolite identification studies in hepatocytes revealed predominant formation of an inactive metabolite (M1) via amide hydrolysis in human. The formation of M1 appeared to be NADPH-independent in human liver microsomes. M1 was found in only minor to moderate quantities in plasma from preclinical species dosed with GDC-0834. Human clearance predictions using various methodologies resulted in estimates ranging from low to high. In addition, GDC-0834 exhibited low clearance in PXB chimeric mice with humanized liver. Uncertainty in human pharmacokinetic prediction and high interest in a BTK inhibitor for clinical evaluation prompted an investigational new drug strategy, in which GDC-0834 was rapidly advanced to a single-dose human clinical trial. GDC-0834 plasma concentrations in humans were below the limit of quantitation (<1 ng/ml) in most samples from the cohorts dosed orally at 35 and 105 mg. In contrast, substantial plasma concentrations of M1 were observed. In human plasma and urine, only M1 and its sequential metabolites were identified. The formation kinetics of M1 was evaluated in rat, dog, monkey, and human liver microsomes in the absence of NADPH. The maximum rate of M1 formation (V(max)) was substantially higher in human compared with that in other species. In contrast, the Michaelis-Menten constant (K(m)) was comparable among species. Intrinsic clearance (V(max)/K(m)) of GDC-0834 from M1 formation in human was 23- to 169-fold higher than observed in rat, dog, and monkey.


