3-Deoxysappanone BCAS# 113122-54-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 113122-54-6 | SDF | Download SDF |
| PubChem ID | 15703606 | Appearance | Powder |
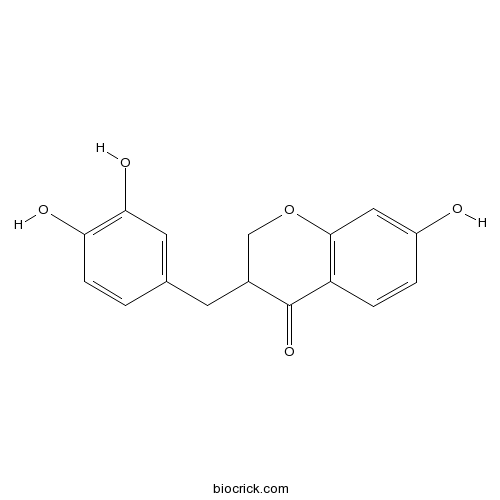
| Formula | C16H14O5 | M.Wt | 286.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[(3,4-dihydroxyphenyl)methyl]-7-hydroxy-2,3-dihydrochromen-4-one | ||
| SMILES | C1C(C(=O)C2=C(O1)C=C(C=C2)O)CC3=CC(=C(C=C3)O)O | ||
| Standard InChIKey | KCUXSQJYIWEGRG-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 3-Deoxysappanone B has vasorelaxation effects, it can mediate endothelium- independent vasodilator action in rat thoracic aortic rings. |
| Targets | NO |

3-Deoxysappanone B Dilution Calculator

3-Deoxysappanone B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4928 mL | 17.4642 mL | 34.9284 mL | 69.8568 mL | 87.321 mL |
| 5 mM | 0.6986 mL | 3.4928 mL | 6.9857 mL | 13.9714 mL | 17.4642 mL |
| 10 mM | 0.3493 mL | 1.7464 mL | 3.4928 mL | 6.9857 mL | 8.7321 mL |
| 50 mM | 0.0699 mL | 0.3493 mL | 0.6986 mL | 1.3971 mL | 1.7464 mL |
| 100 mM | 0.0349 mL | 0.1746 mL | 0.3493 mL | 0.6986 mL | 0.8732 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-O-Methylrosmanol
Catalog No.:BCN7276
CAS No.:113085-62-4
- VU 0155069
Catalog No.:BCC7715
CAS No.:1130067-06-9
- Potassium benzylpenicillin
Catalog No.:BCC9126
CAS No.:113-98-4
- Chlorpheniramine Maleate
Catalog No.:BCC4526
CAS No.:113-92-8
- Chlorprothixene
Catalog No.:BCC3753
CAS No.:113-59-7
- Estradiol diproppionate
Catalog No.:BCC8960
CAS No.:113-38-2
- IM-12
Catalog No.:BCC5487
CAS No.:1129669-05-1
- Tokinolide B
Catalog No.:BCN2753
CAS No.:112966-16-2
- Calcipotriol
Catalog No.:BCC1444
CAS No.:112965-21-6
- PD128907 HCl
Catalog No.:BCC4469
CAS No.:112960-16-4
- HU 211
Catalog No.:BCC5946
CAS No.:112924-45-5
- Boc-D-Asp(OcHex)-OH
Catalog No.:BCC3372
CAS No.:112898-18-7
- (S)-(+)-Niguldipine hydrochloride
Catalog No.:BCC6947
CAS No.:113145-69-0
- Physalin L
Catalog No.:BCN2312
CAS No.:113146-74-0
- nor-Binaltorphimine dihydrochloride
Catalog No.:BCC6614
CAS No.:113158-34-2
- Linopirdine dihydrochloride
Catalog No.:BCC7231
CAS No.:113168-57-3
- PPPA
Catalog No.:BCC7309
CAS No.:113190-92-4
- H-Phe(4-F)-OH
Catalog No.:BCC3216
CAS No.:1132-68-9
- (3R)-Hydrangenol 8-O-glucoside pentaacetate
Catalog No.:BCN1617
CAS No.:113270-98-7
- (3S)-Hydrangenol 8-O-glucoside pentaacetate
Catalog No.:BCN1616
CAS No.:113270-99-8
- ABT-333
Catalog No.:BCC4129
CAS No.:1132935-63-7
- GDC-0834
Catalog No.:BCC5115
CAS No.:1133432-50-4
- neo-Truxilline
Catalog No.:BCN1949
CAS No.:113350-54-2
- γ-Truxilline
Catalog No.:BCN1948
CAS No.:113350-56-4
A new 3-benzylchroman derivative from Sappan Lignum (Caesalpinia sappan).[Pubmed:18794793]
Molecules. 2008 Aug 28;13(8):1923-30.
3'-Deoxy-4-O-methylepisappanol, a new 3-benzylchroman derivative, was isolated from Sappan Lignum, together with thirteen known chemical compounds identified as protosappanin A, sappanchalcone, sappanone B, palmitic acid, (+)-(8S,8'S)-bisdihydrosiringenin, brazilein, 3-deoxysappanchalcone, (+)-lyoniresinol, 3-Deoxysappanone B, protosappanin B, isoprotosappanin B, 3'-O-methylbrazilin and brazilin, respectively. Among these known compounds, this is the first time that (+)-(8S,8'S)-bisdihydrosiringenin was obtained from the family Caesalpiniaceae.
[Vasorelaxation effects of homoisoflavonoids from Caesalpinia sappan in rat thoracic aortic rings].[Pubmed:19624017]
Zhongguo Zhong Yao Za Zhi. 2009 Mar;34(6):731-4.
OBJECTIVE: To identify and elucidate the vasorelaxant activity of homoisoflavonoids, the main chemical components from Lignum Sappan (the stems of Caesalpinia sappan), in isolated rat thoracic aortic rings pre-contracted with phenylephrine (PE, 1 micromol x L(-1)) and KCl (60 mmol x L(-1)). METHOD: The tension of rat thoracic aorta rings was used to evaluated the vasorelaxant activities of four homoisoflavonoids, brazlin (1), (E)-3-(3,4-dihydroxybenzylidene)-7-hydroxychroman-4-one (2), sappanone B (3), 3-Deoxysappanone B (4). RESULT: Cumulative addition of homoisoflavonoids (2, 3 and 4) (50-1000 micromol x L(-1)) exhibited an acute relaxation either in endothelium-intact or endothelium-denuded rings in a concentration-dependent manner. However, this relaxation was significantly inhibited in endothelium-denuded condition and in the presence of endothelial nitric oxide synthase (eNOS) inhibitor, N(W)-nitro-L-arginine methyl ester (L-NNA, 100 micromol x L(-1)), and a soluble guanylate cylcase (sGC) inhibitor, methylene blue (MB, 10 micromol x L(-1)) when addition of variation homoisoflavonoids brazlin (1) (50-1000 micromol x L(-1)). CONCLUSION: These results indicate that normo-homoisoflavonoids (2, 3 and 4) from Caesalpinia sappan mediates endothelium-independent vasodilator action in rat thoracic aortic rings, while the variation homoisoflavonoids brazlin elicits endothelium-dependent relaxation might via nitric oxide (NO)-cGMP pathway. This research could explain the pharmacological activities of homoisoflavonoids to a certain degree.


