PPPACAS# 113190-92-4 |
- BIBR 953 (Dabigatran, Pradaxa)
Catalog No.:BCC2139
CAS No.:211914-51-1
- BIBR-1048
Catalog No.:BCC3738
CAS No.:211915-06-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 113190-92-4 | SDF | Download SDF |
| PubChem ID | 10422256 | Appearance | Powder |
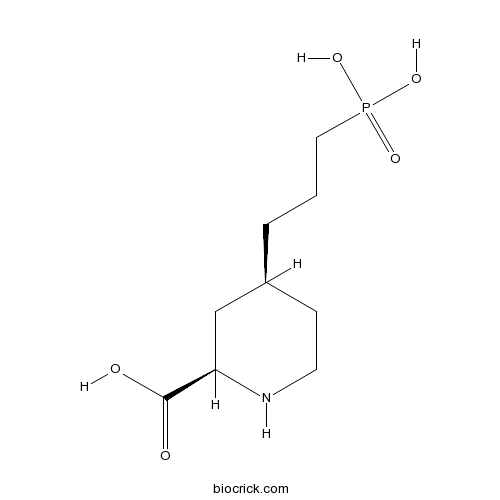
| Formula | C9H18NO5P | M.Wt | 251.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LY 257883 | ||
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | (2R,4S)-4-(3-phosphonopropyl)piperidine-2-carboxylic acid | ||
| SMILES | C1CNC(CC1CCCP(=O)(O)O)C(=O)O | ||
| Standard InChIKey | ABIFUJNCKIMWRZ-JGVFFNPUSA-N | ||
| Standard InChI | InChI=1S/C9H18NO5P/c11-9(12)8-6-7(3-4-10-8)2-1-5-16(13,14)15/h7-8,10H,1-6H2,(H,11,12)(H2,13,14,15)/t7-,8+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Competitive NMDA receptor antagonist that displays moderate selectivity for NR2A-containing receptors (Ki values are 0.13, 0.47, 1.10 and 3.86 μM for NR2A, NR2B, NR2C and NR2D subunits respectively). |

PPPA Dilution Calculator

PPPA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9806 mL | 19.9029 mL | 39.8057 mL | 79.6115 mL | 99.5144 mL |
| 5 mM | 0.7961 mL | 3.9806 mL | 7.9611 mL | 15.9223 mL | 19.9029 mL |
| 10 mM | 0.3981 mL | 1.9903 mL | 3.9806 mL | 7.9611 mL | 9.9514 mL |
| 50 mM | 0.0796 mL | 0.3981 mL | 0.7961 mL | 1.5922 mL | 1.9903 mL |
| 100 mM | 0.0398 mL | 0.199 mL | 0.3981 mL | 0.7961 mL | 0.9951 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Linopirdine dihydrochloride
Catalog No.:BCC7231
CAS No.:113168-57-3
- nor-Binaltorphimine dihydrochloride
Catalog No.:BCC6614
CAS No.:113158-34-2
- Physalin L
Catalog No.:BCN2312
CAS No.:113146-74-0
- (S)-(+)-Niguldipine hydrochloride
Catalog No.:BCC6947
CAS No.:113145-69-0
- 3-Deoxysappanone B
Catalog No.:BCN6012
CAS No.:113122-54-6
- 7-O-Methylrosmanol
Catalog No.:BCN7276
CAS No.:113085-62-4
- VU 0155069
Catalog No.:BCC7715
CAS No.:1130067-06-9
- Potassium benzylpenicillin
Catalog No.:BCC9126
CAS No.:113-98-4
- Chlorpheniramine Maleate
Catalog No.:BCC4526
CAS No.:113-92-8
- Chlorprothixene
Catalog No.:BCC3753
CAS No.:113-59-7
- Estradiol diproppionate
Catalog No.:BCC8960
CAS No.:113-38-2
- IM-12
Catalog No.:BCC5487
CAS No.:1129669-05-1
- H-Phe(4-F)-OH
Catalog No.:BCC3216
CAS No.:1132-68-9
- (3R)-Hydrangenol 8-O-glucoside pentaacetate
Catalog No.:BCN1617
CAS No.:113270-98-7
- (3S)-Hydrangenol 8-O-glucoside pentaacetate
Catalog No.:BCN1616
CAS No.:113270-99-8
- ABT-333
Catalog No.:BCC4129
CAS No.:1132935-63-7
- GDC-0834
Catalog No.:BCC5115
CAS No.:1133432-50-4
- neo-Truxilline
Catalog No.:BCN1949
CAS No.:113350-54-2
- γ-Truxilline
Catalog No.:BCN1948
CAS No.:113350-56-4
- 2-Amino-4-phenylphenol
Catalog No.:BCC8534
CAS No.:1134-36-7
- Baclofen
Catalog No.:BCC8839
CAS No.:1134-47-0
- 2-Aminophenyl phenyl sulfide
Catalog No.:BCC8553
CAS No.:1134-94-7
- Jatamanvaltrate B
Catalog No.:BCN7128
CAS No.:1134138-66-1
- VER 155008
Catalog No.:BCC2338
CAS No.:1134156-31-2
Administration of a probiotic associated with nasal vaccination with inactivated Lactococcus lactis-PppA induces effective protection against pneumoccocal infection in young mice.[Pubmed:20002449]
Clin Exp Immunol. 2010 Mar;159(3):351-62.
Streptococcus pneumoniae is a serious public health problem, especially in developing countries, where available vaccines are not part of the vaccination calendar. We evaluated different respiratory mucosa immunization protocols that included the nasal administration of Lactococcus lactis-pneumococcal protective protein A (PPPA) live, inactivated, and in association with a probiotic (Lc) to young mice. The animals that received Lc by the oral and nasal route presented the highest levels of immunoglobulin (Ig)A and IgG anti-PPPA antibodies in bronchoalveolar lavages (BAL) and IgG in serum, which no doubt contributed to the protection against infection. However, only the groups that received the live and inactivated vaccine associated with the oral administration of the probiotic were able to prevent lung colonization by S. pneumoniae serotypes 3 and 14 in a respiratory infection model. This would be related to a preferential stimulation of the T helper type 1 (Th1) cells at local and systemic levels and with a moderate Th2 and Th17 response, shown by the cytokine profile induced in BAL and by the results of the IgG1/IgG2a ratio at local and systemic levels. Nasal immunization with the inactivated recombinant strain associated with oral Lc administration was able to stimulate the specific cellular and humoral immune response and afford protection against the challenge with the two S. pneumoniae serotypes. The results obtained show the probiotic-inactivated vaccine association as a valuable alternative for application to human health, especially in at-risk populations, and are the first report of a safe and effective immunization strategy using an inactivated recombinant strain.
Connecting type VI secretion, quorum sensing, and c-di-GMP production in fish pathogen Vibrio alginolyticus through phosphatase PppA.[Pubmed:23021863]
Vet Microbiol. 2013 Mar 23;162(2-4):652-62.
Vibrio alginolyticus, a Gram-negative marine bacterium, has brought about severe economic damage to the mariculture industry by causing vibriosis in various fish species. We are intrigued in the regulation of the pathogenesis in this bacterium. Here, we reported a complex regulatory connection among the newly defined type VI secretion system (T6SS), quorum sensing (QS), and 3',5'-cyclic diguanylic acid (c-di-GMP) signal through the phosphatase PPPA encoded in the T6SS gene cluster of V. alginolyticus. Whole-genome transcriptome analysis revealed various regulatory targets of PPPA including the T6SS substrate hemolysin coregulated protein (Hcp), quorum sensing regulator LuxR, exotoxin alkaline serine protease (Asp), flagellar proteins, as well as proteins involved in polysaccharide biosynthesis and transport. Western blot analysis showed PPPA served as a negative regulator of the expression and secretion of Hcp1. Mutation of PPPA resulted in an increased level of the intracellular second messenger c-di-GMP and a decreased expression of the QS regulator LuxR as well as exotoxin Asp. Complementation of intact PPPA gene in DeltaPPPA mutant restored the production of c-di-GMP, LuxR, and Asp to the wild-type level. Phenotypic studies suggested that PPPA takes part in the modulation of biofilm formation, motility, and cell aggregation. These results demonstrated new roles of PPPA in controlling virulence factors and pleiotropic phenotypes and contributed to our understanding of the regulation of pathogenesis in V. alginolyticus.
Transcriptional regulation of the yghJ-pppA-yghG-gspCDEFGHIJKLM cluster, encoding the type II secretion pathway in enterotoxigenic Escherichia coli.[Pubmed:17085567]
J Bacteriol. 2007 Jan;189(1):142-50.
The gene cluster gspCDEFGHIJKLM codes for various structural components of the type II secretion pathway which is responsible for the secretion of heat-labile enterotoxin by enterotoxigenic Escherichia coli (ETEC). In this work, we used a variety of molecular approaches to elucidate the transcriptional organization of the ETEC type II secretion system and to unravel the mechanisms by which the expression of these genes is controlled. We showed that the gspCDEFGHIJKLM cluster and three other upstream genes, yghJ, PPPA, and yghG, are cotranscribed and that a promoter located in the upstream region of yghJ plays a major role in the expression of this 14-gene transcriptional unit. Transcription of the yghJ promoter was repressed 168-fold upon a temperature downshift from 37 degrees C to 22 degrees C. This temperature-induced repression was mediated by the global regulatory proteins H-NS and StpA. Deletion mutagenesis showed that the promoter region encompassing positions -321 to +301 relative to the start site of transcription of yghJ was required for full repression. The yghJ promoter region is predicted to be highly curved and bound H-NS or StpA directly. The binding of H-NS or StpA blocked transcription initiation by inhibiting promoter open complex formation. Unraveling the mechanisms of regulation of type II secretion by ETEC enhances our understanding of the pathogenesis of ETEC and other pathogenic varieties of E. coli.
PppA, a surface-exposed protein of Streptococcus pneumoniae, elicits cross-reactive antibodies that reduce colonization in a murine intranasal immunization and challenge model.[Pubmed:15664941]
Infect Immun. 2005 Feb;73(2):981-9.
The multivalent pneumococcal conjugate vaccine is effective against both systemic disease and otitis media caused by serotypes contained in the vaccine. However, serotypes not covered by the present conjugate vaccine may still cause pneumococcal disease. To address these serotypes, and the remaining otitis media due to Streptococcus pneumoniae, efforts have been devoted to identifying protective protein antigens. Immunity to conserved surface proteins important for adhesion, nutrient acquisition, or other functions could result in a reduction of colonization and a lower disease potential. We have been searching for conserved surface-exposed proteins from S. pneumoniae that may be involved in pathogenesis to test as vaccine candidates. Here, an approximately 20-kDa protein that has significant homology to a nonheme iron-containing ferritin protein from Listeria innocua and other bactoferritins was identified as pneumococcal protective protein A (PPPA). We expressed and purified recombinant PPPA (rPPPA) and evaluated its potential as a vaccine candidate. The antibodies elicited by purified rPPPA were cross-reactive with PPPA from multiple strains of S. pneumoniae and were directed against surface-exposed epitopes. Intranasal immunization of BALB/c mice with PPPA protein and either a synthetic monophosphoryl lipid A analog, RC529AF, or a cholera toxin mutant, CT-E29H, used as an adjuvant reduced nasopharyngeal colonization in mice following intranasal challenge with a heterologous pneumococcal strain. PPPA-specific systemic and local immunoglobulin G (IgG) and IgA antibody responses were induced. The antisera reacted with whole cells of a heterologous S. pneumoniae type 3 strain. These observations indicate that PPPA may be a promising candidate for inclusion in a vaccine against pneumococcal otitis media.
The effect of competitive antagonist chain length on NMDA receptor subunit selectivity.[Pubmed:15721167]
Neuropharmacology. 2005 Mar;48(3):354-9.
The widely-used N-methyl-D-aspartate (NMDA) receptor antagonists (R)-4-(3-phosphonopropyl) piperazine-2-carboxylic acid ((R)-CPP) and (R)-2-amino-7-phosphonoheptanoate ((R)-AP7) are frequently used as general NMDA receptor antagonists and assumed not to display significant selectivity among NMDA receptor NR2 subunits. However, electrophysiological studies have suggested that certain longer chain N-methyl-D-aspartate (NMDA) receptor competitive antagonists, such as (R)-CPP are ineffective at subpopulations of NMDA receptors in the red nucleus, superior colliculus, and hippocampus. Using recombinant receptors expressed in Xenopus oocytes, we have examined the effect of antagonist chain length on NR2 subunit selectivity. All antagonists displayed the potency order (high to low affinity) of NR2A > NR2B > NR2C > NR2D, however the longer chain antagonists (having 7 instead of 5 bond lengths between acidic groups) displayed much greater subunit selectivity than their short-chain homologues. For example (R)-CPP displayed a 50-fold difference in affinity between NR2A-containing and NR2D-containing NMDA receptors, while the shorter chain homologue 4-(phosphonomethyl) piperazine-2-carboxylic acid (PMPA) displayed only a 5-fold variation in affinity. These results can account for the earlier physiological findings and suggest that longer chain antagonists such as (R)-CPP and (R)-AP7 should not be used as general NMDA receptor antagonists.
Identification of subunit- and antagonist-specific amino acid residues in the N-Methyl-D-aspartate receptor glutamate-binding pocket.[Pubmed:15743930]
J Pharmacol Exp Ther. 2005 Jun;313(3):1066-74.
The resolved X-ray crystal structures of the glutamate-binding domain (S1/S2 domains) of the GluR2 and NR1 glutamate receptor subunits were used to model the homologous regions of the N-methyl-D-aspartate (NMDA) receptor's NR2 subunits. To test the predictive value of these models, all four stereoisomers of the antagonist 1-(phenanthren-2-carbonyl) piperazine-2,3-dicarboxylic acid (PPDA) were docked into the NR2B glutamate-binding site model. This analysis suggested an affinity order for the PPDA isomers of d-cis > L-cis > L-trans = D-trans and predicted that the 2-position carboxylate group of the cis-PPDA isomers, but not of the trans-PPDA isomers, may be interacting with histidine 486 in NR2B. Consistent with these predictions, cis-PPDA displays a 35-fold higher affinity for NR2B-containing NMDA receptors than trans-PPDA. In addition, mutating NR2B's H486 to phenylalanine decreased cis-PPDA affinity 8-fold but had no effect on trans-PPDA affinity. In contrast, the NR2B H486F mutation increased the affinity of the typical antagonists CGS-19755 [(2R*,4S*)-4-phosphonomethyl-2-piperidine carboxylic acid] and 4-(3-phosphonopropyl) piperidine-2-carboxylic acid. In the NR1-based NR2 models, there were only four subunit-specific amino acid residues exposed to the ligand-binding pocket (and six in the GluR2-based models). These residues are located at the edge of the binding pocket, suggesting that large antagonists may be necessary for subtype specificity. Of these residues, mutational analysis and modeling suggest that A414, R712, and G713 (NR2B numbering) may be especially useful for developing NR2C- and NR2D-selective NMDA receptor antagonists and that residues A414 and T428 may determine subunit variations in agonist affinity.
Effect of extracellular pH on the potency of N-methyl-D-aspartic acid receptor competitive antagonists.[Pubmed:1435743]
Mol Pharmacol. 1992 Oct;42(4):679-86.
Structure-activity analysis reveals that acidic alpha-amino acids containing an omega-PO3H2 group are more potent antagonists at N-methyl-D-aspartate (NMDA) receptors than are analogs with omega-COOH or omega-tetrazole groups. At physiological values of extra-cellular pH the omega-PO3H2 group is only partially deprotonated and the corresponding antagonists exist as ions with one or two negative charges. In contrast, competitive antagonists with omega-COOH and omega-tetrazole groups are fully ionized at physiological pH but carry only a single negative charge. Dose-inhibition analysis was performed with (2R)-AP7 and its piperidine derivative LY 257883 to determine whether ionization of the omega-PO3H2 group influences NMDA receptor antagonist potency; these experiments revealed a > 3-fold increase in potency on raising of the extracellular pH from 7.3 to pH 8.2, consistent with the increase in the relative concentration of the ionic form of the antagonist in which the omega-PO3H2 group contains two negative charges. Experiments with the omega-COOH-containing analog of LY 257883 and with SDZ EAB 515, an omega-PO3H2-containing antagonist of novel structure, revealed only 1.5- and 1.3-fold increases in potency, respectively, over the same pH range. Analysis of the kinetics of block of NMDA-activated currents resulting from rapid application of LY 257883 suggests that the increase in potency on raising of the extracellular pH results largely from an increase in the antagonist association rate constant but also from a small decrease in the dissociation rate constant. Together, these results suggest that the fully ionized forms of the R-enantiomers of AP7 and LY 257883 act as the active antagonist species at NMDA receptors.


