BIBR-1048Thrombin inhibitor CAS# 211915-06-9 |
- Bivalirudin Trifluoroacetate
Catalog No.:BCC1421
CAS No.:128270-60-0
- Dabigatran ethyl ester
Catalog No.:BCC1512
CAS No.:429658-95-7
- 5-R-Rivaroxaban
Catalog No.:BCC1313
CAS No.:865479-71-6
- Dabigatran etexilate mesylate
Catalog No.:BCC1511
CAS No.:872728-81-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 211915-06-9 | SDF | Download SDF |
| PubChem ID | 6445226 | Appearance | Powder |
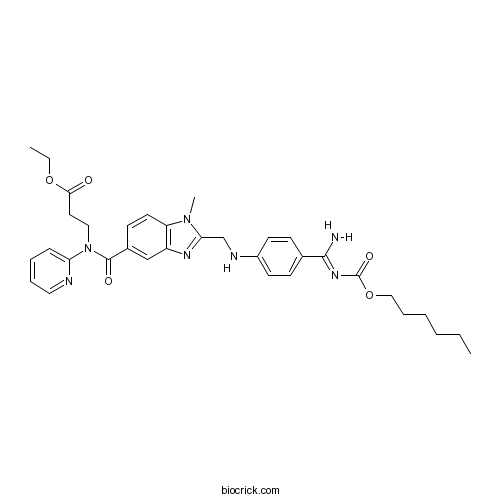
| Formula | C34H41N7O5 | M.Wt | 627.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BIBR 1048 | ||
| Solubility | DMSO : ≥ 100 mg/mL (159.30 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | ethyl 3-[[2-[[4-[(Z)-N'-hexoxycarbonylcarbamimidoyl]anilino]methyl]-1-methylbenzimidazole-5-carbonyl]-pyridin-2-ylamino]propanoate | ||
| SMILES | CCCCCCOC(=O)N=C(N)c1ccc(NCc2nc3cc(ccc3n2C)C(=O)N(CCC(=O)OCC)c4ccccn4)cc1 | ||
| Standard InChIKey | KSGXQBZTULBEEQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C34H41N7O5/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dabigatran etexilate(BIBR-1048) is the orally active prodrug of dabigatran; Dabigatran is a reversible and selective, direct thrombin inhibitor (DTI) with Ki value of 4.5 nM.
IC50 Value: 4.5 nM (Ki); 10 nM(Thrombin-induced platelet aggregation) [1]
in vitro: Dabigatran selectively and reversibly inhibited human thrombin(Ki: 4.5 nM) as well as thrombin-induced platelet aggregation (IC(50): 10 nM), while showing no inhibitory effect on other platelet-stimulating agents.Thrombin generation in platelet-poor plasma (PPP), measured as the endogenous thrombin potential (ETP) was inhibited concentration-dependently (IC(50): 0.56 microM). Dabigatran demonstrated concentration-dependent anticoagulant effects in various species in vitro, doubling the activated partial thromboplastin time (aPTT), prothrombin time (PT) and ecarin clotting time (ECT) in human PPP at concentrations of 0.23, 0.83 and 0.18 microM, respectively [1].
in vivo: Dabigatran prolonged the aPTT dose-dependently after intravenous administration in rats (0.3, 1 and 3 mg/kg) and rhesus monkeys (0.15, 0.3 and 0.6 mg/kg). Dose- and time-dependent anticoagulant effects were observed with dabigatran etexilate administered orally to conscious rats (10, 20 and 50 mg/kg) or rhesus monkeys (1, 2.5 or 5 mg/kg), with maximum effects observed between 30 and 120 min after administration, respectively [1]. Patients treated with dabigatran etexilate experienced fewer ischaemic strokes (3.74 dabigatran etexilate vs 3.97 warfarin) and fewer combined intracranial haemorrhages and haemorrhagic strokes (0.43 dabigatran etexilate vs 0.99 warfarin) per 100 patient-years [2].
Clinical trial: An Evaluation of the Pharmacokinetics and Pharmacodynamics of Oral Dabigatran Etexilate in Hemodialysis Patients . Phase1 References: | |||||

BIBR-1048 Dilution Calculator

BIBR-1048 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.593 mL | 7.9652 mL | 15.9304 mL | 31.8608 mL | 39.826 mL |
| 5 mM | 0.3186 mL | 1.593 mL | 3.1861 mL | 6.3722 mL | 7.9652 mL |
| 10 mM | 0.1593 mL | 0.7965 mL | 1.593 mL | 3.1861 mL | 3.9826 mL |
| 50 mM | 0.0319 mL | 0.1593 mL | 0.3186 mL | 0.6372 mL | 0.7965 mL |
| 100 mM | 0.0159 mL | 0.0797 mL | 0.1593 mL | 0.3186 mL | 0.3983 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dabigatran etexilate is a potent, selective and competitive inhibitor of thrombin. It is an oral prodrug of dabigatran. Ki=4.5 nM for human thrombin and IC50=10 nM for thrombin-induced platelet aggregation. [1] [2]
Thrombin is protein that proteolytically cleaved from coagulation factor II as the initial step in the coagulation cascade. It transforms fibrinogen into fibrin and activates other factors in function in blood homeostasis, wound healing and inflammation.
In vitro, dabigatran showed anticoagulant effects in a concentration-dependent manner. It doubled the activated partrial thromboplastin time, prothrombin time and ecarin clotting in human PPP. [1]
In rats and rhesus monkeys, activated partial throboplastin time was extended by dabigaran.
Rats orally administrated with dabigatran etexilate exerted anticoagulant effects in a dose and time –dependent manner. [1]
Compared with warfarin, dabigatran administered orally (150 mg dose) in patients with atrial fibraillation, exhibited lower rates of stroke and systemic embolism but similar rates of major hemorrhage. [2]
References:
1. Wienen W, Stassen JM, Priepke H et al. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost. 2007 Jul;98(1):155-62.
2. Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial
fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139-51.
- BIBR 953 (Dabigatran, Pradaxa)
Catalog No.:BCC2139
CAS No.:211914-51-1
- Gliclazide
Catalog No.:BCC5002
CAS No.:21187-98-4
- Flumorph
Catalog No.:BCC5467
CAS No.:211867-47-9
- Nudicaucin A
Catalog No.:BCN7842
CAS No.:211815-97-3
- 2',4',5'-Trimethoxy-2'',2''-dimethylpyrano[5'',6'':6,7]isoflavone
Catalog No.:BCN1496
CAS No.:211799-56-3
- Picroside IV
Catalog No.:BCN6533
CAS No.:211567-04-3
- Nudicaucin B
Catalog No.:BCN7843
CAS No.:211557-36-7
- WHI-P180
Catalog No.:BCC3928
CAS No.:211555-08-7
- WHI-P97
Catalog No.:BCC2056
CAS No.:211555-05-4
- WHI-P154
Catalog No.:BCC2202
CAS No.:211555-04-3
- Dalcetrapib (JTT-705, RO4607381)
Catalog No.:BCC2328
CAS No.:211513-37-0
- Dendrobine
Catalog No.:BCN5923
CAS No.:2115-91-5
- 1,3,7-Trihydroxy-2-methoxyxanthone
Catalog No.:BCN7549
CAS No.:211948-69-5
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Vatalanib
Catalog No.:BCC2085
CAS No.:212141-54-3
- Apparicine
Catalog No.:BCN4008
CAS No.:2122-36-3
- 5,7-Dihydroxy-3-(4-hydroxy-3,5-dimethoxybenzyl)-6,8-dimethylchroman-4-one
Catalog No.:BCN6631
CAS No.:212201-12-2
- Ipfencarbazone
Catalog No.:BCC5465
CAS No.:212201-70-2
- Ethyl 3-(3-amino-4-(methylamino)-N-(pyridin-2-yl)benzamido)propanoate
Catalog No.:BCC8971
CAS No.:212322-56-0
- TC 2559 difumarate
Catalog No.:BCC7469
CAS No.:212332-35-9
- HS 024
Catalog No.:BCC5820
CAS No.:212370-59-7
- Oxaprozin
Catalog No.:BCC9109
CAS No.:21256-18-8
- Ebracteolatanolide A
Catalog No.:BCN3773
CAS No.:212563-72-9
- Nocistatin (human)
Catalog No.:BCC5732
CAS No.:212609-11-5
BIBR-1048 Boehringer Ingelheim.[Pubmed:12137410]
Curr Opin Investig Drugs. 2002 Jun;3(6):905-7.
BIBR-1048, a thrombin inhibitor and an orally-active prodrug of BIBR-953ZW, is under development by Boehringer Ingelheim as a potential antithrombotic agent [331881]. By 1999, BIBR-1048 was in phase II clinical trials for thromboembolism and the prevention of stroke due to atrial fibrillation [331881]; by April 2002, proof-of-principle had been demonstrated in phase II trials in deep vein thrombosis [446554]. In July 2001, the company revealed that an IND was expected to be filed for BIBR-953ZW in 2002 [415884].
Population pharmacokinetic analysis of the new oral thrombin inhibitor dabigatran etexilate (BIBR 1048) in patients undergoing primary elective total hip replacement surgery.[Pubmed:17322149]
J Clin Pharmacol. 2007 Mar;47(3):371-82.
Dabigatran etexilate (BIBR 1048) is an orally bioavailable double prodrug of the active principle dabigatran (BIBR 953 ZW), which exerts potent anticoagulant and antithrombotic activity. The objective of the analysis was to develop a population pharmacokinetic model characterizing and quantifying the relationship between covariates and model parameters. A total of 4604 BIBR 953 ZW plasma concentrations, obtained from 287 patients after once- or twice-daily oral dosing for up to 10 days after surgery in the dose range 12.5, 25, 50, 100, 150, 200, and 300 mg BIBR 1048, were available for the analysis. All the analyses were performed with NONMEN V. Pharmacokinetics of dabigatran were best described by a 2-compartment model. The data supported the estimation of different apparent first-order absorption rate constants (k(a)) and apparent plasma clearances (CL/F) for days 0 and 1 and days 2 to 10 after surgery. Parameter estimates indicated a flip-flop phenomenon. Age and serum creatinine influenced k(a), whereas gastrin and creatinine clearance, only for days 2 to 10, affected CL/F (P < .001). The typical values for CL/F for a patient with gastrin of 34.58 pmol/L and creatinine clearance of 76.16 mL/min were 70.87 and 106.2 L/h on days 0 and 1 and days 2 to 10, respectively. The differences found in the pharmacokinetics of dabigatran during the first 24 hours after surgery are most likely due to alterations in gastric motility and pH following surgery. As a consequence, the rate of absorption is reduced and interindividual variability in drug exposure increased. On the following days, the disposition in plasma of BIBR 953 ZW is less variable.


