WHI-P180EGFR/Janus Kinase 3 inhibitor CAS# 211555-08-7 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 211555-08-7 | SDF | Download SDF |
| PubChem ID | 5687 | Appearance | Powder |
| Formula | C16H15N3O3 | M.Wt | 297.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Janex 3 | ||
| Solubility | DMSO : 25 mg/mL (84.09 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
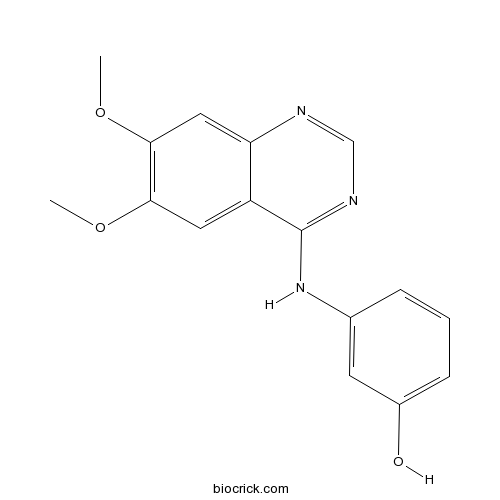
| Chemical Name | 3-[(6,7-dimethoxyquinazolin-4-yl)amino]phenol | ||
| SMILES | COC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC(=CC=C3)O)OC | ||
| Standard InChIKey | BNDYIYYKEIXHNK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H15N3O3/c1-21-14-7-12-13(8-15(14)22-2)17-9-18-16(12)19-10-4-3-5-11(20)6-10/h3-9,20H,1-2H3,(H,17,18,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | WHI-P180 (Janex 3) is a multi-kinase inhibitor; inhibits RET, KDR and EGFR with IC50s of 5 nM, 66 nM and 4 μM, respectively.In Vivo:WHI-P180 is also an active inhibitor of IgE-mediated mast cell responses. The elimination half-life of WHI-P180 in CD-1 mice (BALB/c mice) following i.v., i.p., or p.o. administration is less than 10 min. Systemic clearance of WHI-P180 is 6742 mL/h/kg in CD-I mice and 8188 mL/h/kg in BALB/c mice. Notably, WHI-P180, when administered in two consecutive nontoxic i.p. bolus doses of 25 mg/kg, inhibits IgE/antigen-induced vascular hyperpermeability in a well-characterized murine model of passive cutaneous anaphylaxis[3]. References: | |||||

WHI-P180 Dilution Calculator

WHI-P180 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3635 mL | 16.8175 mL | 33.6349 mL | 67.2699 mL | 84.0873 mL |
| 5 mM | 0.6727 mL | 3.3635 mL | 6.727 mL | 13.454 mL | 16.8175 mL |
| 10 mM | 0.3363 mL | 1.6817 mL | 3.3635 mL | 6.727 mL | 8.4087 mL |
| 50 mM | 0.0673 mL | 0.3363 mL | 0.6727 mL | 1.3454 mL | 1.6817 mL |
| 100 mM | 0.0336 mL | 0.1682 mL | 0.3363 mL | 0.6727 mL | 0.8409 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
WHI-P180 is a potent inhibitors [with IC50 = 4.0 microM for epidermal growth factor receptor (EGFR) kinase inhibition] of the EGFR tyrosine kinase as well as Janus Kinase 3. Besides, WHI-P180 is a potent inhibitor of lgE-mediated mast cell responses to al
- WHI-P97
Catalog No.:BCC2056
CAS No.:211555-05-4
- WHI-P154
Catalog No.:BCC2202
CAS No.:211555-04-3
- Dalcetrapib (JTT-705, RO4607381)
Catalog No.:BCC2328
CAS No.:211513-37-0
- Dendrobine
Catalog No.:BCN5923
CAS No.:2115-91-5
- Astrocasine
Catalog No.:BCN2150
CAS No.:2114-92-3
- R18
Catalog No.:BCC2383
CAS No.:211364-78-2
- m-Chlorophenylbiguanide hydrochloride
Catalog No.:BCC6650
CAS No.:2113-05-5
- 9,17-Octadecadiene-12,14-diyne-1,11,16-triol
Catalog No.:BCN1497
CAS No.:211238-60-7
- Rubranol
Catalog No.:BCN4917
CAS No.:211126-61-3
- Sobetirome
Catalog No.:BCC1957
CAS No.:211110-63-3
- Marsformoxide B
Catalog No.:BCN6687
CAS No.:2111-46-8
- SB 265610
Catalog No.:BCC5936
CAS No.:211096-49-0
- Nudicaucin B
Catalog No.:BCN7843
CAS No.:211557-36-7
- Picroside IV
Catalog No.:BCN6533
CAS No.:211567-04-3
- 2',4',5'-Trimethoxy-2'',2''-dimethylpyrano[5'',6'':6,7]isoflavone
Catalog No.:BCN1496
CAS No.:211799-56-3
- Nudicaucin A
Catalog No.:BCN7842
CAS No.:211815-97-3
- Flumorph
Catalog No.:BCC5467
CAS No.:211867-47-9
- Gliclazide
Catalog No.:BCC5002
CAS No.:21187-98-4
- BIBR 953 (Dabigatran, Pradaxa)
Catalog No.:BCC2139
CAS No.:211914-51-1
- BIBR-1048
Catalog No.:BCC3738
CAS No.:211915-06-9
- 1,3,7-Trihydroxy-2-methoxyxanthone
Catalog No.:BCN7549
CAS No.:211948-69-5
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Vatalanib
Catalog No.:BCC2085
CAS No.:212141-54-3
- Apparicine
Catalog No.:BCN4008
CAS No.:2122-36-3
Treatment of post-bone marrow transplant acute graft-versus-host disease with a rationally designed JAK3 inhibitor.[Pubmed:12389628]
Leuk Lymphoma. 2002 Jul;43(7):1447-53.
Here we show that the Janus kinase 3 (JAK3) inhibitor 4-(3'-hydroxyphenyl)-amino-6,7-dimethoxyquinazoline (JANEX-3) exhibits potent anti-GVHD activity and consequently improves the post-BMT survival outcome of C57BL/6 (H-2b) recipient mice transplanted with allogeneic bone marrow/splenocyte (BM/S) grafts from MHC disparate BALB/c mice (H-2d). One hundred percent of the vehicle-treated allograft recipients developed severe GVHD and died with a median survival of 41 days. Treatment of recipient mice with JANEX-3 (30 mg/kg/day, 3 x/day) after the onset of rapidly progressive severe GVHD in the 3rd week after BMT significantly improved the survival of BMT recipients with GVHD and prolonged the median survival time to 78 days (P < 0.0001, log-rank test). The probability of survival at two and three months post-BMT was 6 +/- 6% and 0 +/- 0% for vehicle-treated control mice and 100 +/- 0% and 38 +/- 17% for mice treated with JANEX-3. These results prompted the hypothesis that JAK3 plays a pivotal role in the pathophysiology of GVHD. To test this hypothesis, we examined if mice transplanted with allogeneic BM/S grafts from Jak3 knockout mice Jak3-/- develop GVHD. The allografts from (Jak3-/-) C57BL/6 (H-2b) mice rescued MHC-disparate recipient BALB/c mice (H-2d) of the lethal toxicity of TBI without causing fatal GVHD. Taken together, these observations establish JAK3 as a key mediator of severe GVHD after allogeneic BMT in the context of a major-HLA disparity.
Global kinase screening. Applications of frontal affinity chromatography coupled to mass spectrometry in drug discovery.[Pubmed:15732906]
Anal Chem. 2005 Mar 1;77(5):1268-74.
Utilizing frontal affinity chromatography with mass spectrometry detection (FAC-MS), we have identified novel applications in the discovery of small-molecule hits to protein targets that are difficult if not impossible to accomplish using traditional assays. We demonstrate for the first time an ability to distinguish between competitive ligands for the ATP and substrate sites of protein kinase C independently in the same experiment and show that ATP competitive ligands using a functionally inactive receptor tyrosine kinase can be identified. This ability of FAC-MS to simultaneously monitor binding at the ATP and substrate binding sites, as well as measure ligand binding to both active and inactive kinases, suggests that FAC-MS can be used as a "global kinase binding assay".
Pharmacokinetics and biologic activity of the novel mast cell inhibitor, 4-(3-hydroxyphenyl)-amino-6,7-dimethoxyquinazoline in mice.[Pubmed:9950289]
Pharm Res. 1999 Jan;16(1):117-22.
PURPOSE: The purpose of the present study was to examine the pharmacodynamic and pharmacokinetic features of the novel mast cell inhibitor 4-(3'-Hydroxyphenyl)-amino-6,7-dimethoxyquinazoline (WHI-P180) in mice. METHODS: A high performance liquid chromatography (HPLC)-based quantitative detection method was used to measure plasma WHI-P180 levels in mice. The plasma concentration-time data was fit to a single compartment pharmacokinetic model by using the WinNonlin program to calculate the pharmacokinetic parameters. A cutaneous anaphylaxis model was used to examine the pharmacodynamic effects of WHI-P180 on anaphylaxis-associated vascular hyperpermeability. RESULTS: The elimination half-life of WHI-P180 in CD-1 mice (BALB/ c mice) following i.v., i.p., or p.o. administration was less than 10 min. Systemic clearance of WHI-P180 was 6742 mL/h/kg in CD-I mice and 8188 mL/h/kg in BALB/c mice. Notably, WHI-P180, when administered in two consecutive nontoxic i.p. bolus doses of 25 mg/kg, inhibited IgE/antigen-induced vascular hyperpermeability in a well-characterized murine model of passive cutaneous anaphylaxis. CONCLUSIONS: WHI-P180 is an active inhibitor of IgE-mediated mast cell responses in vitro and in vivo. Further preclinical characterization of WHI-P180 may improve the efficacy of WHI-P180 in vivo and provide the basis for design of effective treatment and prevention programs for mast cell mediated allergic reactions.
4-[3-Bromo-4-hydroxyphenyl)amino]-6,7-dimethoxyquinazolin-1-ium chloride methanol solvate and 4-[(3-hydroxyphenyl)amino]-6,7-dimethoxy-1-quinazolinium chloride.[Pubmed:11173405]
Acta Crystallogr C. 2001 Jan;57(Pt 1):76-8.
The title compounds, C16H15BrN3(O3)(+).Cl(-).CH4O (WHI-P154) and C16H16N3(O3)(+).Cl(-) (WHI-P180), are potent inhibitors [WHI-P154 with IC50 = 5.6 microM and WHI-P180 with IC50 = 4.0 microM for epidermal growth factor receptor (EGFR) kinase inhibition] of the EGFR tyrosine kinase as well as Janus Kinase 3. The molecular structures of these compounds are very similar except for the dihedral angle between the anilino and quinazoline moieties which is 1.10 (5) degrees for WHI-P154, and 45.66 (6) and 25.29 (7) degrees for the two molecules of WHI-P180 in the asymmetric unit. The nitrogen at the N3 position is protonated in both structures and participates in hydrogen bonding with the chlorine anions.
Cellular phototoxicity evoked through the inhibition of human ABC transporter ABCG2 by cyclin-dependent kinase inhibitors in vitro.[Pubmed:18841444]
Pharm Res. 2009 Feb;26(2):449-58.
PURPOSE: The physiological importance of the human ATP-binding cassette (ABC) transporter ABCG2 has been recognized with regard to porphyrin-mediated photosensitivity. Functional impairment owing to inhibition of ABCG2 by drugs or its genetic polymorphisms may lead to the disruption of porphyrin homeostasis, which in turn causes cellular toxicity. MATERIALS AND METHODS: We evaluated the impact on photosensitivity of the inhibition by cyclin-dependent kinase (CDK) inhibitors of ABCG2 function. For this purpose, we established new methods for photosensitivity assays by using Flp-In-293 cells and plasma membrane vesicles prepared from Sf9 insect cells. With the new methods, we subsequently tested CDK inhibitors, i.e., purvalanol A, WHI-P180, bohemine, roscovitine, and olomoucine. RESULTS: Among CDK inhibitors tested, purvalanol A was found to be the most potent inhibitor (IC50=3.5 microM) for ABCG2-mediated hematoporphyrin transport. At a concentration of 2.5 microM, it evoked the photosensitivity of ABCG2-expressing Flp-In-293 cells treated with pheophorbide a. WHI-P180 moderately inhibited ABCG2 function, exhibiting weak phototoxicity. In contrast, the phototoxicity of bohemine, roscovitine, and olomoucine were minimal in our assay system. CONCLUSIONS: It is suggested that the planar structure is an important factor for interactions with the active site of ABCG2. The present study provides a new approach to studying drug-induced phototoxicity in vitro.


