Nu 6027ATR/CDK inhibitor, potent and selective CAS# 220036-08-8 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- AT7519 trifluoroacetate
Catalog No.:BCC1377
CAS No.:1431697-85-6
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 220036-08-8 | SDF | Download SDF |
| PubChem ID | 398148 | Appearance | Powder |
| Formula | C11H17N5O2 | M.Wt | 251.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
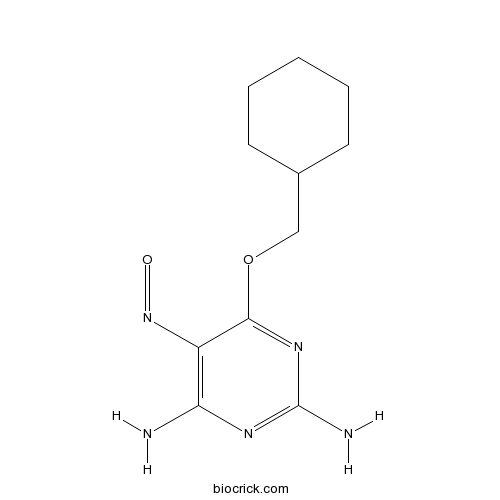
| Chemical Name | 6-(cyclohexylmethoxy)-5-nitrosopyrimidine-2,4-diamine | ||
| SMILES | C1CCC(CC1)COC2=NC(=NC(=C2N=O)N)N | ||
| Standard InChIKey | DGWXOLHKVGDQLN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H17N5O2/c12-9-8(16-17)10(15-11(13)14-9)18-6-7-4-2-1-3-5-7/h7H,1-6H2,(H4,12,13,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nu 6027 is a selective inhibitor of CDK1 and CDK2 with IC50 value of 2.9 and 2.2 µM, respectively. | |||||
| Targets | CDK1 | CDK2 | ||||
| IC50 | 2.9 µM | 2.2 µM | ||||

Nu 6027 Dilution Calculator

Nu 6027 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9796 mL | 19.8981 mL | 39.7962 mL | 79.5925 mL | 99.4906 mL |
| 5 mM | 0.7959 mL | 3.9796 mL | 7.9592 mL | 15.9185 mL | 19.8981 mL |
| 10 mM | 0.398 mL | 1.9898 mL | 3.9796 mL | 7.9592 mL | 9.9491 mL |
| 50 mM | 0.0796 mL | 0.398 mL | 0.7959 mL | 1.5918 mL | 1.9898 mL |
| 100 mM | 0.0398 mL | 0.199 mL | 0.398 mL | 0.7959 mL | 0.9949 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NU6027 is a potent inhibitor of cellular ATR activity with the IC50 of 6.7 μM.
The ataxia telangiectasia mutated and Rad3-related kinase (ATR) has a crucial role in the stalled replication forks signalling and DNA damage to cell cycle checkpoints and DNA repair. ATR was recognised as an key target for cancer therapy, however, its inhibitors have proved elusive. NU6027, which was originally developed as a CDK2 inhibitor, inhibits ATR.
In vitro: NU6027 is a potent inhibitor of cellular ATR activity with the IC50 of 6.7 μM and enhanced the cytotoxicity of hydroxyurea and cisplatin in an ATR-dependent manner. NU6027 attenuated G2/M phase arrest with DNA damage, inhibited the formation of RAD51 focus and increased the cytotoxicity of the major classes of DNA-damaging cytotoxic therapy but not paclitaxel and antimitotic. In A2780 cells, cisplatin sensitisation was greatest in cells with functional p53 and mismatch repair and sensitisation to temozolomide was greatest in p53 mutant cells with functional mismatch repair. More importantly, NU6027 was found to be synthetically lethal when DNA single-strand break repair is impaired either through poly(ADP-ribose) polymerase (PARP) inhibition or defects in XRCC1 [1].
In vivo: Currently no in-vivo data are available.
Clinical trial: NU6027 is still in preclinical development stage and no clinicl trial is ongoing currently.
Reference:
[1] Peasland A, Wang LZ, Rowling E, Kyle S, Chen T, Hopkins A, Cliby WA, Sarkaria J, Beale G, Edmondson RJ, Curtin NJ. Identification and evaluation of a potent novel ATR inhibitor, NU6027, in breast and ovarian cancer cell lines. Br J Cancer. 2011;105(3):372-81.
- Ixabepilone
Catalog No.:BCC1666
CAS No.:219989-84-1
- Fmoc-β-Homo-Tyr(tBu)-OH
Catalog No.:BCC2621
CAS No.:219967-69-8
- Isoescin IB
Catalog No.:BCN2969
CAS No.:219944-46-4
- Isoescin IA
Catalog No.:BCN2968
CAS No.:219944-39-5
- 3-Methyl-1-(2-piperidinophenyl)butylamine N-acetylglutamate salt
Catalog No.:BCC8635
CAS No.:219921-94-5
- 5,8,9,14-Tetraacetoxy-3-benzoyloxy-10,15-dihydroxypepluane
Catalog No.:BCN7657
CAS No.:219916-77-5
- MPEP Hydrochloride
Catalog No.:BCC1777
CAS No.:219911-35-0
- 2,2',3'-Trihydroxy-4,6-dimethoxybenzophenone
Catalog No.:BCN1488
CAS No.:219861-73-1
- Escitalopram Oxalate
Catalog No.:BCC5040
CAS No.:219861-08-2
- Merresectine B
Catalog No.:BCN1918
CAS No.:219829-75-1
- Consiculine
Catalog No.:BCN1903
CAS No.:219829-73-9
- BTB06584
Catalog No.:BCC5106
CAS No.:219793-45-0
- Vincetoxicoside B
Catalog No.:BCN2864
CAS No.:22007-72-3
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Eichlerialactone
Catalog No.:BCN4941
CAS No.:2202-01-9
- O-Methyldauricine
Catalog No.:BCC8225
CAS No.:2202-17-7
- Triptohairic acid
Catalog No.:BCN8060
CAS No.:220209-71-2
- Cinnamamide
Catalog No.:BCN4942
CAS No.:22031-64-7
- 5-[2-[Tert-butyl(dimethyl)silyl]oxyethyl]-2,2-dimethyl-3a,6a-dihydrofuro[2,3-d][1,3]dioxol-6-one
Catalog No.:BCC8592
CAS No.:220328-03-0
- 3,11,12-Trihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1487
CAS No.:220328-04-1
- TRO 19622
Catalog No.:BCC5288
CAS No.:22033-87-0
- DH 97
Catalog No.:BCC6973
CAS No.:220339-00-4
- 5-Epicanadensene
Catalog No.:BCN7349
CAS No.:220384-17-8
- ShK-Dap22
Catalog No.:BCC5990
CAS No.:220384-25-8
Lambda_{c}-->Lambdal^{+}nu_{l} Form Factors and Decay Rates from Lattice QCD with Physical Quark Masses.[Pubmed:28282163]
Phys Rev Lett. 2017 Feb 24;118(8):082001.
The first lattice QCD calculation of the form factors governing Lambda_{c}-->Lambdal^{+}nu_{l} decays is reported. The calculation was performed with two different lattice spacings and includes one ensemble with a pion mass of 139(2) MeV. The resulting predictions for the Lambda_{c}-->Lambdae^{+}nu_{e} and Lambda_{c}-->Lambdamu^{+}nu_{mu} decay rates divided by |V_{cs}|^{2} are 0.2007(71)(74) and 0.1945(69)(72) ps^{-1}, respectively, where the two uncertainties are statistical and systematic. Taking the Cabibbo-Kobayashi-Maskawa (CKM) matrix element |V_{cs}| from a global fit and the Lambda_{c} lifetime from experiments, this translates to branching fractions of B(Lambda_{c}-->Lambdae^{+}nu_{e})=0.0380(19)_{LQCD}(11)_{tau_{Lambda_{c}}} and B(Lambda_{c}-->Lambdamu^{+}nu_{mu})=0.0369(19)_{LQCD}(11)_{tau_{Lambda_{c}}}. These results are consistent with, and two times more precise than, the measurements performed recently by the BESIII Collaboration. Using instead the measured branching fractions together with the lattice calculation to determine the CKM matrix element gives |V_{cs}|=0.949(24)_{LQCD}(14)_{tau_{Lambda_{c}}}(49)_{B}.
An Experimental Study of the Kinetics of OH/OD(v = 1,2,3) + SO2: The Limiting High-Pressure Rate Coefficients as a Function of Temperature.[Pubmed:28363245]
J Phys Chem A. 2017 May 4;121(17):3175-3183.
The kinetics of the reaction OH/OD(v = 1,2,3) + SO2 were studied using a photolysis/laser-induced fluorescence technique. The rate coefficients OH/OD(v = 1,2,3) + SO2, k1, over the temperature range of 295-810 K were used to determine the limiting high-pressure limit k1(infinity). This method is usually applicable if the reaction samples the potential well of the adduct HOSO2 and if intramolecular vibrational relaxation is fast. In the present case, however, the rate coefficients showed an additional fast removal contribution as evidenced by the increase in k1 with vibrational level; this behavior together with its temperature dependence is consistent with the existence of a weakly bound complex on the potential energy surface prior to adduct formation. The data were analyzed using a composite mechanism that incoporates energy-transfer mechanisms via both the adduct and the complex, and yielded a value of k1(infinity)(295 K) equal to (7.2 +/- 3.3) x 10(-13) cm(3) molecule(-1) s(-1) (errors at 1sigma), a factor of between 2 and 3 smaller than the current recommended IUPAC and JPL values of (2.0-1.0(+2.0)) and (1.6 +/- 0.4) x 10(-12) cm(3) molecule(-1) s(-1) at 298 K, respectively, although the error bars do overlap. k1(infinity) was observed to only depend weakly on temperature. Further evidence for a smaller k1(infinity) is presented in the companion paper.
Investigation of Image Reconstruction Parameters of the Mediso nanoScan PC Small-Animal PET/CT Scanner for Two Different Positron Emitters Under NEMA NU 4-2008 Standards.[Pubmed:27995432]
Mol Imaging Biol. 2017 Aug;19(4):550-559.
PURPOSE: The Tera-Tomo 3D image reconstruction algorithm (a version of OSEM), provided with the Mediso nanoScan(R) PC (PET8/2) small-animal positron emission tomograph (PET)/x-ray computed tomography (CT) scanner, has various parameter options such as total level of regularization, subsets, and iterations. Also, the acquisition time in PET plays an important role. This study aims to assess the performance of this new small-animal PET/CT scanner for different acquisition times and reconstruction parameters, for 2-deoxy-2-[(18)F]fluoro-D-glucose ([(18)F]FDG) and Ga-68, under the NEMA NU 4-2008 standards. PROCEDURES: Various image quality metrics were calculated for different realizations of [(18)F]FDG and Ga-68 filled image quality (IQ) phantoms. RESULTS: [(18)F]FDG imaging produced improved images over Ga-68. The best compromise for the optimization of all image quality factors is achieved for at least 30 min acquisition and image reconstruction with 52 iteration updates combined with a high regularization level. CONCLUSION: A high regularization level at 52 iteration updates and 30 min acquisition time were found to optimize most of the figures of merit investigated.
Insights into Supramolecular Sites Responsible for Complete Separation of Biomass-Derived Phenolics and Glucose in Metal-Organic Framework NU-1000.[Pubmed:28296411]
Langmuir. 2017 May 2;33(17):4129-4137.
The molecular origins of adsorption of lignin-derived phenolics to metal-organic framework NU-1000 are investigated from aqueous solution as well as in competitive mode with glucose present in the same aqueous mixture. A comparison of adsorption equilibrium constants (Kads) for phenolics functionalized with either carboxylic acid or aldehyde substituents demonstrated only a slight increase (less than a factor of 6) for the former according to both experiments and calculations. This small difference in Kads between aldehyde and carboxylic-acid substituted adsorbates is consistent with the pyrene unit of NU-1000 as the adsorption site, rather than the zirconia nodes, while at saturation coverage, the adsorption capacity suggests multiple guests per pyrene. Experimental standard free energies of adsorption directly correlated with the molecular size and electronic structure calculations confirmed this direct relationship, with the pyrene units as adsorption site. The underlying origins of this relationship are grounded in noncovalent pi-pi interactions as being responsible for adsorption, the same interactions present in the condensed phase of the phenolics, which to a large extent govern their heat of vaporization. Thus, NU-1000 acts as a preformed aromatic cavity for driving aromatic guest adsorption from aqueous solution and does so specifically without causing detectable glucose adsorption from aqueous solution, thereby achieving complete glucose-phenolics separations. The reusability of NU-1000 during an adsorption/desorption cycle was good, even with some of the phenolic compounds with greatest affinity not easiliy removed with water and ethanol washes at room temperature. A competitive adsorption experiment gave an upper bound for Kads for glucose of at most 0.18 M(-1), which can be compared with Kads for the phenolics investigated here, which fell in the range of 443-42639 M(-1). The actual value of Kads for glucose may be much closer to zero given the lack of observed glucose uptake with NU-1000 as adsorbent.


