MPEP HydrochlorideMGlu5 receptor antagonist CAS# 219911-35-0 |
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- mGlu2 agonist
Catalog No.:BCC1745
CAS No.:1311385-32-6
- LY341495
Catalog No.:BCC1724
CAS No.:201943-63-7
- CPPHA
Catalog No.:BCC1501
CAS No.:693288-97-0
- Dipraglurant
Catalog No.:BCC1531
CAS No.:872363-17-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 219911-35-0 | SDF | Download SDF |
| PubChem ID | 9794588 | Appearance | Powder |
| Formula | C14H12ClN | M.Wt | 229.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (217.68 mM) *"≥" means soluble, but saturation unknown. | ||
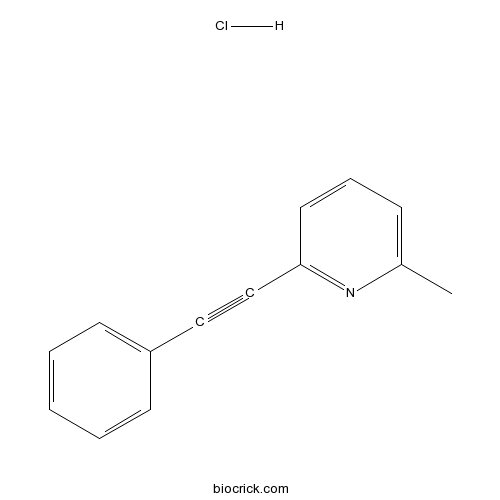
| Chemical Name | 2-methyl-6-(2-phenylethynyl)pyridine;hydrochloride | ||
| SMILES | CC1=CC=CC(=N1)C#CC2=CC=CC=C2.Cl | ||
| Standard InChIKey | PKDHDJBNEKXCBI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H11N.ClH/c1-12-6-5-9-14(15-12)11-10-13-7-3-2-4-8-13;/h2-9H,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and highly selective non-competitive antagonist at the mGlu5 receptor subtype (IC50 = 36 nM) and a positive allosteric modulator at mGlu4 receptors. Centrally active following systemic administration in vivo. Reverses mechanical hyperalgesia in the inflamed rat hind paw. Also available as part of the Group I mGlu Receptor. |

MPEP Hydrochloride Dilution Calculator

MPEP Hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3535 mL | 21.7675 mL | 43.535 mL | 87.0701 mL | 108.8376 mL |
| 5 mM | 0.8707 mL | 4.3535 mL | 8.707 mL | 17.414 mL | 21.7675 mL |
| 10 mM | 0.4354 mL | 2.1768 mL | 4.3535 mL | 8.707 mL | 10.8838 mL |
| 50 mM | 0.0871 mL | 0.4354 mL | 0.8707 mL | 1.7414 mL | 2.1768 mL |
| 100 mM | 0.0435 mL | 0.2177 mL | 0.4354 mL | 0.8707 mL | 1.0884 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 36 nM
MPEP is a potent, selective and systemically active mGlu5 receptor antagonist.
Metabotropic glutamate (mGlu) receptors are a of G-protein-coupled receptor family linked to multiple second messengers and modulation of ion channel functions in the nervous system. Group I receptors (mGlu1 and -5) couple to phospholipase C and up or down regulate neuronal excitability (Gereau and Conn, 1995) whereas group II (mGlu2 and -3) and group III (mGlu4, -6, -7, and -8) receptors inhibit adenylyl cyclase and reduce synaptic transmission.
In vitro: In recombinant expressed cells human mGlu5a receptor, MPEP inhibited quisqualate-stimulated phosphoinositide hydrolysis completely with an IC50 value of 36 nM whereas with no agonist or antagonist activities at cells expressing the human mGlu1b receptor. MPEP did not show agonist or antagonist activity on human mGlu2, -3, -4a, -7b, and -8a receptors [1].
In vivo: In rat neonatal brain slices, MPEP inhibited DHPG-stimulated phosphoinositide hydrolysis with a potency and selectivity similar to that observed on human mGlu receptors. Moreover, in extracellular recordings in the hippocampus CA1 area of anesthetized rats, the microiontophoretic application of DHPG induced neuronal firing that was blocked when MPEP was administered [1].
Clinical trial: N/A
Reference:
[1] Gasparini F,Lingenhhl K,Stoehr N,Flor PJ,Heinrich M,Vranesic I,Biollaz M,Allgeier H,Heckendorn R,Urwyler S,Varney MA,Johnson EC,Hess SD,Rao SP,Sacaan AI,Santori EM,Velielebi G,Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology.1999 Oct;38(10):1493-503.
- 2,2',3'-Trihydroxy-4,6-dimethoxybenzophenone
Catalog No.:BCN1488
CAS No.:219861-73-1
- Escitalopram Oxalate
Catalog No.:BCC5040
CAS No.:219861-08-2
- Merresectine B
Catalog No.:BCN1918
CAS No.:219829-75-1
- Consiculine
Catalog No.:BCN1903
CAS No.:219829-73-9
- BTB06584
Catalog No.:BCC5106
CAS No.:219793-45-0
- Bombiprenone
Catalog No.:BCN4940
CAS No.:21978-49-4
- ANA 12
Catalog No.:BCC6287
CAS No.:219766-25-3
- Taxezopidine L
Catalog No.:BCN6946
CAS No.:219749-76-5
- 14-Deoxy-12-hydroxyandrographolide
Catalog No.:BCN4673
CAS No.:219721-33-2
- Baicalin
Catalog No.:BCN5901
CAS No.:21967-41-9
- Griffipavixanthone
Catalog No.:BCN4939
CAS No.:219649-95-3
- (R)-Coclaurine
Catalog No.:BCN8348
CAS No.:2196-60-3
- 5,8,9,14-Tetraacetoxy-3-benzoyloxy-10,15-dihydroxypepluane
Catalog No.:BCN7657
CAS No.:219916-77-5
- 3-Methyl-1-(2-piperidinophenyl)butylamine N-acetylglutamate salt
Catalog No.:BCC8635
CAS No.:219921-94-5
- Isoescin IA
Catalog No.:BCN2968
CAS No.:219944-39-5
- Isoescin IB
Catalog No.:BCN2969
CAS No.:219944-46-4
- Fmoc-β-Homo-Tyr(tBu)-OH
Catalog No.:BCC2621
CAS No.:219967-69-8
- Ixabepilone
Catalog No.:BCC1666
CAS No.:219989-84-1
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- Vincetoxicoside B
Catalog No.:BCN2864
CAS No.:22007-72-3
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Eichlerialactone
Catalog No.:BCN4941
CAS No.:2202-01-9
- O-Methyldauricine
Catalog No.:BCC8225
CAS No.:2202-17-7
- Triptohairic acid
Catalog No.:BCN8060
CAS No.:220209-71-2
Early-Onset Network Hyperexcitability in Presymptomatic Alzheimer's Disease Transgenic Mice Is Suppressed by Passive Immunization with Anti-Human APP/Abeta Antibody and by mGluR5 Blockade.[Pubmed:28392767]
Front Aging Neurosci. 2017 Mar 24;9:71.
Cortical and hippocampal network hyperexcitability appears to be an early event in Alzheimer's disease (AD) pathogenesis, and may contribute to memory impairment. It remains unclear if network hyperexcitability precedes memory impairment in mouse models of AD and what are the underlying cellular mechanisms. We thus evaluated seizure susceptibility and hippocampal network hyperexcitability at ~3 weeks of age [prior to amyloid beta (Abeta) plaque deposition, neurofibrillary pathology, and cognitive impairment] in a triple transgenic mouse model of familial AD (3xTg-AD mouse) that harbors mutated human Abeta precursor protein (APP), tau and presenilin 1 (PS1) genes. Audiogenic seizures were elicited in a higher proportion of 3xTg-AD mice compared with wild type (WT) controls. Seizure susceptibility in 3xTg-AD mice was attenuated either by passive immunization with anti-human APP/Abeta antibody (6E10) or by blockade of metabotropic glutamate receptor 5 (mGluR5) with the selective antagonist, 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP). In in vitro hippocampal slices, suppression of synaptic inhibition with the GABAA receptor antagonist, bicuculline, induced prolonged epileptiform (>1.5 s in duration) ictal-like discharges in the CA3 neuronal network in the majority of the slices from 3xTg-AD mice. In contrast, only short epileptiform (<1.5 s in duration) interictal-like discharges were observed following bicuculline application in the CA3 region of WT slices. The ictal-like activity in CA3 region of the hippocampus was significantly reduced in the 6E10-immunized compared to the saline-treated 3xTg-AD mice. MPEP acutely suppressed the ictal-like discharges in 3xTg-AD slices. Remarkably, epileptiform discharge duration positively correlated with intraneuronal human (transgenic) APP/Abeta expression in the CA3 region of the hippocampus. Our data suggest that in a mouse model of familial AD, hypersynchronous network activity underlying seizure susceptibility precedes Abeta plaque pathology and memory impairment. This early-onset network hyperexcitability can be suppressed by passive immunization with an anti-human APP/Abeta antibody and by mGluR5 blockade in 3xTg-AD mice.
2-Methyl-6-(phenylethynyl)pyridine Hydrochloride Modulates Metabotropic Glutamate 5 Receptors Endogenously Expressed in Zebrafish Brain.[Pubmed:27635438]
ACS Chem Neurosci. 2016 Dec 21;7(12):1690-1697.
Due to phylogenetic proximity to the human, zebrafish has been recognized as a reliable model to study Alzheimer's disease (AD) and other central nervous system disorders. Furthermore, metabotropic glutamate receptors have been previously reported to be impaired in brain from AD patients. Metabotropic glutamate 5 (mGlu5) receptors are G-protein coupled receptors proposed as potential targets for therapy of different neurodegenerative disorders. Thus, MPEP (2-methyl-6-(phenylethynyl)pyridine hydrochloride), a selective noncompetitive mGlu5 receptor antagonist, has been suggested for pharmacological treatment of AD. The aim of the present work was to quantify mGlu5 receptors in brain from zebrafish and to study the possible modulation of these receptors by MPEP treatment. To this end, radioligand binding assay and open field test were used. Results showed a slightly higher presence of mGlu5 receptors in brain from male than in that from female zebrafish. However, a significant increase of mGlu5 receptor in male without variation in female was observed after MPEP treatment. This gender specific response was also observed in locomotor behavior, being significantly decreased only in male zebrafish. These results confirm the presence of mGlu5 receptors in brain from zebrafish and their gender specific modulation by selective antagonist treatment and suggest a role of these receptors on locomotor activity, which is affected in many disorders. In addition, our data point to zebrafish as a useful model to study mGlu receptor function in both healthy and pathological conditions.
DSR-98776, a novel selective mGlu5 receptor negative allosteric modulator with potent antidepressant and antimanic activity.[Pubmed:25823809]
Eur J Pharmacol. 2015 Jun 15;757:11-20.
Modulation of monoaminergic systems has been the main stream of treatment for patients with mood disorders. However, recent evidence suggests that the glutamatergic system plays an important role in the pathophysiology of these disorders. This study pharmacologically characterized a structurally novel metabotropic glutamate 5 (mGlu5) receptor negative allosteric modulator, DSR-98776, and evaluated its effect on rodent models of depression and mania. First, DSR-98776 in vitro profile was assessed using intracellular calcium and radioligand binding assays. This compound showed dose-dependent inhibitory activity for mGlu5 receptors by binding to the same allosteric site as 2-methyl-6-(phenylethynyl)-pyridine (MPEP), a known mGlu5 inhibitor. The in vivo therapeutic benefits of DSR-98776 were evaluated in common rodent models of depression and mania. In the rat forced swimming test, DSR-98776 (1-3mg/kg) significantly reduced rats immobility time after treatment for 7 consecutive days, while paroxetine (3 and 10mg/kg) required administration for 2 consecutive weeks to reduce rats immobility time. In the mouse forced swimming test, acute administration of DSR-98776 (10-30 mg/kg) significantly reduced immobility time. This effect was not influenced by 4-chloro-DL-phenylalanine methyl ester hydrochloride-induced 5-HT depletion. Finally, DSR-98776 (30 mg/kg) significantly decreased methamphetamine/chlordiazepoxide-induced hyperactivity in mice, which reflects this compound antimanic-like effect. These results indicate that DSR-98776 acts as an orally potent antidepressant and antimanic in rodent models and can be a promising therapeutic option for the treatment of a broad range of mood disorders with depressive and manic states.
Acute inhibition of mGluR5 disrupts behavioral flexibility.[Pubmed:26777993]
Neurobiol Learn Mem. 2016 Apr;130:1-6.
Conditioned cues can sometimes elicit maladaptive responses as seen in the post-traumatic stress disorder (PTSD). Lack of effective fear extinction, which involves a process of inhibitory learning, is hypothesized to associate with PTSD. In this study, we tested the effect of acute pharmacological inhibition of mGluR5 activity on the extinction of fear memory and spatial memory. Intraperitoneal injection of the mGluR5 (metabotropic glutamate receptor 5) antagonist MPEP [2-Methyl-6-(phenylethynyl) pyridine hydrochloride] allowed the retrieval but prevented the extinction of contextual fear memory in mice. Without altering locomotor activity, MPEP inhibited the acquisition but not the consolidation of contextual fear memory. Further, administration of MPEP blocked the extinction of spatial memory in the Morris water maze paradigm. Our data suggest a necessary role of mGluR5 in regulating certain aspects of behavioral flexibility.
Estradiol Facilitation of Cocaine Self-Administration in Female Rats Requires Activation of mGluR5.[Pubmed:27822496]
eNeuro. 2016 Oct 25;3(5). pii: eN-NWR-0140-16.
In comparison to men, women initiate drug use at earlier ages and progress from initial use to addiction more rapidly. This heightened intake and vulnerability to drugs of abuse is regulated in part by estradiol, although the signaling mechanisms by which this occurs are not well understood. Recent findings indicate that within the nucleus accumbens core, estradiol induces structural plasticity via membrane-localized estrogen receptor alpha, functionally coupled to metabotropic glutamate receptor subtype 5 (mGluR5). Hence, we sought to determine whether mGluR5 activation was essential for estradiol-mediated enhancement of cocaine self-administration. Ovariectomized (OVX) female rats were allowed to freely self-administer cocaine under extended access conditions (6 h/d) for 10 consecutive days. The mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) or vehicle was administered before estradiol (or oil), on a 2 d on/2 d off schedule throughout the extended access period. MPEP treatment prevented the estradiol-dependent enhancement of cocaine self-administration in OVX females. In a separate experiment, potentiation of mGluR5 function with the positive allosteric modulator 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (in the absence of estradiol treatment) failed to increase cocaine self-administration. These data suggest that mGluR5 activation is necessary for estradiol-mediated enhancement of responses to cocaine, but that direct mGluR5 activation is insufficient to mimic the female response to estradiol. Building on previous studies in male animals, these findings further highlight the therapeutic potential of mGluR5 antagonism in the treatment of addiction and suggest that there may be added therapeutic benefit in females.
Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP.[Pubmed:12684257]
Br J Pharmacol. 2003 Mar;138(6):1026-30.
We have identified 2-methyl-6-(2-phenylethenyl)pyridine (SIB-1893) and 2-methyl-6-phenylethynyl pyridine hydrochloride (MPEP) as positive allosteric modulators for the hmGluR4. SIB-1893 and MPEP enhanced the potency and efficacy of L-2-amino-4-phophonobutyrate (L-AP4) in guanosine 5'-O-(3-[(35)S]thiotriphosphate ([(35)S]GTPgammaS) binding and efficacy in cAMP studies. These effects were fully blocked by the mGluR4 competitive antagonist (RS)-alpha-cyclopropyl-4-phosphonophenylglycine (CPPG), indicating a dependency on receptor activation. Although SIB-1893 and MPEP had no effects alone in GTPgammaS binding, effects were observed in the cell-based cAMP assay due to media-derived activation as indicated by CPPG inhibition. Positive modulation of the mGluR4 was a receptor-specific effect since SIB-1893 and MPEP had neither effects on mGluR2-expressing cells nor on the parent BHK cell line. In [(3)H]L-AP4 binding, a two-fold decrease in K(D) but not in B(max) was observed with 100 micro M SIB-1893, whereas MPEP affected neither parameter. Finally, SIB-1893 and MPEP failed to displace [(3)H]L-AP4 binding. Taken together, these data identify positive allosteric modulators for the hmGluR4.
2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist.[Pubmed:10530811]
Neuropharmacology. 1999 Oct;38(10):1493-503.
In the present paper we describe 2-methyl-6-(phenylethynyl)-pyridine (MPEP) as a potent, selective and systemically active antagonist for the metabotropic glutamate receptor subtype 5 (mGlu5). At the human mGlu5a receptor expressed in recombinant cells, MPEP completely inhibited quisqualate-stimulated phosphoinositide (PI) hydrolysis with an IC50 value of 36 nM while having no agonist or antagonist activities at cells expressing the human mGlu1b receptor at concentrations up to 30 microM. When tested at group II and III receptors, MPEP did not show agonist or antagonist activity at 100 microM on human mGlu2, -3, -4a, -7b, and -8a receptors nor at 10 microM on the human mGlu6 receptor. Electrophysiological recordings in Xenopus laevis oocytes demonstrated no significant effect at 100 microM on human NMDA (NMDA1A/2A), rat AMPA (Glu3-(flop)) and human kainate (Glu6-(IYQ)) receptor subtypes nor at 10 microM on the human NMDA1A/2B receptor. In rat neonatal brain slices, MPEP inhibited DHPG-stimulated PI hydrolysis with a potency and selectivity similar to that observed on human mGlu receptors. Furthermore, in extracellular recordings in the CA1 area of the hippocampus in anesthetized rats, the microiontophoretic application of DHPG induced neuronal firing that was blocked when MPEP was administered by iontophoretic or intravenous routes. Excitations induced by microiontophoretic application of AMPA were not affected.
Antagonism of the mGlu5 agonist 2-chloro-5-hydroxyphenylglycine by the novel selective mGlu5 antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) in the thalamus.[Pubmed:10455248]
Br J Pharmacol. 1999 Jul;127(5):1057-9.
Our previous work has shown that Group I mGlu receptors participate in thalamic sensory processing in vivo. However, unequivocal demonstration of mGlu5 participation has not been possible due to the lack of specific ligands. We have therefore made a preliminary study of the in vivo actions of the agonist (R,S)-2-Chloro-5-hydroxyphenylglycine [CHPG] and the novel mGlu5 antagonist 6-methyl-2-(phenylethynyl)-pyridine [MPEP] in order to characterize their suitability for functional studies. Iontophoretically administered MPEP selectively antagonized excitatory responses of single rat thalamic neurones to CHPG compared to the broad-spectrum mGlu agonist (1S,3R)-1-aminocyclopentane-1,3-dicarboxylate. In contrast, the established mGlu1 and mGlu5 antagonist (S)-4-carboxyphenylglycine reduced responses to both agonists. These findings are the first demonstration of an in vivo action of CHPG and its antagonism by a selective mGlu5 antagonist. Furthermore MPEP appears to be a good tool for functional studies of mGlu5.


