FlumorphCAS# 211867-47-9 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 211867-47-9 | SDF | Download SDF |
| PubChem ID | 11057755 | Appearance | Powder |
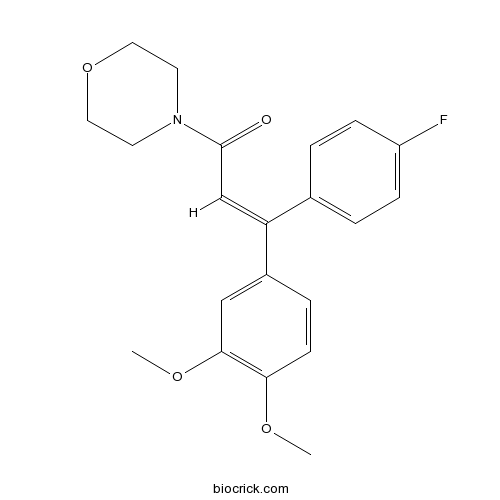
| Formula | C21H22FNO4 | M.Wt | 371.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | (E)-3-(3,4-dimethoxyphenyl)-3-(4-fluorophenyl)-1-morpholin-4-ylprop-2-en-1-one | ||
| SMILES | COC1=C(C=C(C=C1)C(=CC(=O)N2CCOCC2)C3=CC=C(C=C3)F)OC | ||
| Standard InChIKey | BKBSMMUEEAWFRX-NBVRZTHBSA-N | ||
| Standard InChI | InChI=1S/C21H22FNO4/c1-25-19-8-5-16(13-20(19)26-2)18(15-3-6-17(22)7-4-15)14-21(24)23-9-11-27-12-10-23/h3-8,13-14H,9-12H2,1-2H3/b18-14+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Flumorph Dilution Calculator

Flumorph Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6925 mL | 13.4626 mL | 26.9251 mL | 53.8503 mL | 67.3129 mL |
| 5 mM | 0.5385 mL | 2.6925 mL | 5.385 mL | 10.7701 mL | 13.4626 mL |
| 10 mM | 0.2693 mL | 1.3463 mL | 2.6925 mL | 5.385 mL | 6.7313 mL |
| 50 mM | 0.0539 mL | 0.2693 mL | 0.5385 mL | 1.077 mL | 1.3463 mL |
| 100 mM | 0.0269 mL | 0.1346 mL | 0.2693 mL | 0.5385 mL | 0.6731 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Flumorph(SYP-L190) is a carboxylic acid amide (CAA) fungicide.
- Nudicaucin A
Catalog No.:BCN7842
CAS No.:211815-97-3
- 2',4',5'-Trimethoxy-2'',2''-dimethylpyrano[5'',6'':6,7]isoflavone
Catalog No.:BCN1496
CAS No.:211799-56-3
- Picroside IV
Catalog No.:BCN6533
CAS No.:211567-04-3
- Nudicaucin B
Catalog No.:BCN7843
CAS No.:211557-36-7
- WHI-P180
Catalog No.:BCC3928
CAS No.:211555-08-7
- WHI-P97
Catalog No.:BCC2056
CAS No.:211555-05-4
- WHI-P154
Catalog No.:BCC2202
CAS No.:211555-04-3
- Dalcetrapib (JTT-705, RO4607381)
Catalog No.:BCC2328
CAS No.:211513-37-0
- Dendrobine
Catalog No.:BCN5923
CAS No.:2115-91-5
- Astrocasine
Catalog No.:BCN2150
CAS No.:2114-92-3
- R18
Catalog No.:BCC2383
CAS No.:211364-78-2
- m-Chlorophenylbiguanide hydrochloride
Catalog No.:BCC6650
CAS No.:2113-05-5
- Gliclazide
Catalog No.:BCC5002
CAS No.:21187-98-4
- BIBR 953 (Dabigatran, Pradaxa)
Catalog No.:BCC2139
CAS No.:211914-51-1
- BIBR-1048
Catalog No.:BCC3738
CAS No.:211915-06-9
- 1,3,7-Trihydroxy-2-methoxyxanthone
Catalog No.:BCN7549
CAS No.:211948-69-5
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Vatalanib
Catalog No.:BCC2085
CAS No.:212141-54-3
- Apparicine
Catalog No.:BCN4008
CAS No.:2122-36-3
- 5,7-Dihydroxy-3-(4-hydroxy-3,5-dimethoxybenzyl)-6,8-dimethylchroman-4-one
Catalog No.:BCN6631
CAS No.:212201-12-2
- Ipfencarbazone
Catalog No.:BCC5465
CAS No.:212201-70-2
- Ethyl 3-(3-amino-4-(methylamino)-N-(pyridin-2-yl)benzamido)propanoate
Catalog No.:BCC8971
CAS No.:212322-56-0
- TC 2559 difumarate
Catalog No.:BCC7469
CAS No.:212332-35-9
- HS 024
Catalog No.:BCC5820
CAS No.:212370-59-7
DNA damage and effects on antioxidative enzymes in earthworm (Eisenia fetida) induced by flumorph.[Pubmed:24352618]
Appl Biochem Biotechnol. 2014 Feb;172(4):2276-85.
Flumorph is an Oomycete fungicide, which is used extensively as an effective fungicide in vegetables and fruits, but little is known about its effect on nontarget soil organisms. In the present study, biochemical responses including changes in the activity of antioxidative enzymes catalase (CAT), superoxide dismutase (SOD), glutathione-S-transferase (GST), malondialdehyde (MDA), and DNA damage induced by Flumorph were investigated in earthworms (Eisenis fetida). The CAT concentrations were stimulated at 5.0 mg kg(-1) over 28 days and inhibited at 10 and 20 mg kg(-1), except 10 mg kg(-1) on days 21 and 28 compared with the controls. The overall SOD activities were inhibited except 5 mg kg(-1) on day 28 and 10 mg kg(-1) on days 7 and 14. Meanwhile, the GST activities were stimulated on day 7 and decreased on the other days in summary. The MDA activities were increased notably at 5, 10, and 20 mg kg(-1) after 14 days. Clear dose-dependent DNA damage to Eisenia fetida was observed by olive tail moments in comet assay compared with controls. The results demonstrate that Flumorph induces oxidative stress and DNA damage to earthworms, and the effects may be the important mechanisms of its toxicity.
[Determination of flumorph and dimethomorph residues in vegetables by improved QuEChERS-gas chromatography-mass spectromery].[Pubmed:25434121]
Se Pu. 2014 Aug;32(8):849-54.
To determine the residues of Flumorph and dimethomorph in vegetables, a method was established with improved QuEChERS-gas chromatography coupled to mass spectrometry (GC-MS). With acetonitrile as the extraction solvent, the samples were pretreated with the improved QuEChERS method including extraction, salting-out and purification processes. Then all the sample extracts were analyzed with GC-MS in selected-ion monitoring (SIM) mode, and quantified by matrix-matched standard solution in external standard method. Under electron ionization conditions, the analysis was carried out with a capillary column (DB-5 MS, 30 mx 0. 25 mm x 0. 25 mum) at a flow rate of 1. 1 mL/min. The quantitation was performed to detect ions of m/z 285, 371, 165 for Flumorph and m/z 301, 387, 165 for dimethomorph. Good linearity was obtained in the range of 10-1 000 mug/kg for both pesticides with correlation coefficients greater than 0. 999. The recovery experiments were carried out by spiking blank samples of ginger, tomato, carrot, spinach, cabbage and tremella at the three levels of 10, 20 and 100 mug/kg. For both pesticides in different matrices, the limits of detection (S/N=3) were in the range of 0. 67-2. 42 mug/kg. The average recoveries of Flumorph and dimethomorph ranged from 71% to 116% with the relative standard deviations (RSDs) in the range of 1. 8%- 14. 7%. Meanwhile, the pyrolysis mechanism and matrix effect for the determination of Flumorph and dimethomorph in vegetables were investigated in this study. The method is simple, rapid and accurate, and can be used for the routine analysis of Flumorph and dimethomorph in vegetables.
Insights into the adaptive response of the plant-pathogenic oomycete Phytophthora capsici to the fungicide flumorph.[Pubmed:27050922]
Sci Rep. 2016 Apr 6;6:24103.
Phytophthora capsici is an important oomycete plant pathogen that causes significant losses worldwide. The carboxylic acid amide fungicide Flumorph has shown excellent activity against oomycete plant pathogens. Despite its potential, there remains concern that the sexual reproduction of oomycete pathogens, which results in genetic recombination, could result in the rapid development of resistance to Flumorph. The current study utilized an iTRAQ (isobaric tags for relative and absolute quantitation) based method to compare differences between the proteome of the parental P. capsici isolate PCAS1 and its sexual progeny S2-838, which exhibits significant resistance to Flumorph. A total of 2396 individual proteins were identified, of these, 181 were considered to be associated with the adaptive response of P. capsici to Flumorph. The subsequent bioinformatic analysis revealed that the adaptive response of P. capsici to Flumorph was complex and regulated by multiple mechanisms, including utilising carbohydrate from the host environment to compensate for the cell wall stress induced by Flumorph, a shift in energy generation, decreased amino acids biosynthesis, and elevated levels of proteins associated with the pathogen's response to stimulus and transmembrane transport. Moreover, the results of the study provided crucial data that could provide the basis for early monitoring of Flumorph resistance in field populations of P. capsici.
Effect of Flumorph on F-Actin Dynamics in the Potato Late Blight Pathogen Phytophthora infestans.[Pubmed:25496300]
Phytopathology. 2015 Apr;105(4):419-23.
Oomycetes are fungal-like pathogens that cause notorious diseases. Protecting crops against oomycetes requires regular spraying with chemicals, many with an unknown mode of action. In the 1990s, Flumorph was identified as a novel crop protection agent. It was shown to inhibit the growth of oomycete pathogens including Phytophthora spp., presumably by targeting actin. We recently generated transgenic Phytophthora infestans strains that express Lifeact-enhanced green fluorescent protein (eGFP), which enabled us to monitor the actin cytoskeleton during hyphal growth. For analyzing effects of oomicides on the actin cytoskeleton in vivo, the P. infestans Lifeact-eGFP strain is an excellent tool. Here, we confirm that Flumorph is an oomicide with growth inhibitory activity. Microscopic analyses showed that low Flumorph concentrations provoked hyphal tip swellings accompanied by accumulation of actin plaques in the apex, a feature reminiscent of tips of nongrowing hyphae. At higher concentrations, swelling was more pronounced and accompanied by an increase in hyphal bursting events. However, in hyphae that remained intact, actin filaments were indistinguishable from those in nontreated, nongrowing hyphae. In contrast, in hyphae treated with the actin depolymerizing drug latrunculin B, no hyphal bursting was observed but the actin filaments were completely disrupted. This difference demonstrates that actin is not the primary target of Flumorph.


