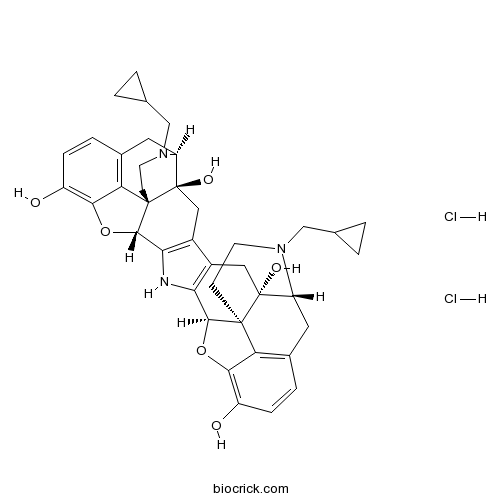
nor-Binaltorphimine dihydrochlorideCAS# 113158-34-2 |
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- Tezampanel
Catalog No.:BCC1993
CAS No.:154652-83-2
- LY450108
Catalog No.:BCC1725
CAS No.:376594-67-1
- Perampanel
Catalog No.:BCC1847
CAS No.:380917-97-5
- Aniracetam
Catalog No.:BCC4219
CAS No.:72432-10-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 113158-34-2 | SDF | Download SDF |
| PubChem ID | 11957626 | Appearance | Powder |
| Formula | C40H45Cl2N3O6 | M.Wt | 734.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | <em>nor</em>-BNI, Norbinaltorphimine | ||
| Solubility | Soluble to 25 mM in water with gentle warming and to 25 mM in DMSO | ||
| SMILES | C1CC1CN2CCC34C5C6=C(CC3(C2CC7=C4C(=C(C=C7)O)O5)O)C8=C(N6)C9C12CCN(C(C1(C8)O)CC1=C2C(=C(C=C1)O)O9)CC1CC1.Cl.Cl | ||
| Standard InChIKey | JOJPJLHRMGPDPV-LZQROVCBSA-N | ||
| Standard InChI | InChI=1S/C40H43N3O6.2ClH/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19;;/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2;2*1H/t27-,28-,35+,36+,37+,38+,39-,40-;;/m1../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A selective κ-opioid receptor antagonist. |

nor-Binaltorphimine dihydrochloride Dilution Calculator

nor-Binaltorphimine dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3611 mL | 6.8053 mL | 13.6106 mL | 27.2213 mL | 34.0266 mL |
| 5 mM | 0.2722 mL | 1.3611 mL | 2.7221 mL | 5.4443 mL | 6.8053 mL |
| 10 mM | 0.1361 mL | 0.6805 mL | 1.3611 mL | 2.7221 mL | 3.4027 mL |
| 50 mM | 0.0272 mL | 0.1361 mL | 0.2722 mL | 0.5444 mL | 0.6805 mL |
| 100 mM | 0.0136 mL | 0.0681 mL | 0.1361 mL | 0.2722 mL | 0.3403 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Physalin L
Catalog No.:BCN2312
CAS No.:113146-74-0
- (S)-(+)-Niguldipine hydrochloride
Catalog No.:BCC6947
CAS No.:113145-69-0
- 3-Deoxysappanone B
Catalog No.:BCN6012
CAS No.:113122-54-6
- 7-O-Methylrosmanol
Catalog No.:BCN7276
CAS No.:113085-62-4
- VU 0155069
Catalog No.:BCC7715
CAS No.:1130067-06-9
- Potassium benzylpenicillin
Catalog No.:BCC9126
CAS No.:113-98-4
- Chlorpheniramine Maleate
Catalog No.:BCC4526
CAS No.:113-92-8
- Chlorprothixene
Catalog No.:BCC3753
CAS No.:113-59-7
- Estradiol diproppionate
Catalog No.:BCC8960
CAS No.:113-38-2
- IM-12
Catalog No.:BCC5487
CAS No.:1129669-05-1
- Tokinolide B
Catalog No.:BCN2753
CAS No.:112966-16-2
- Calcipotriol
Catalog No.:BCC1444
CAS No.:112965-21-6
- Linopirdine dihydrochloride
Catalog No.:BCC7231
CAS No.:113168-57-3
- PPPA
Catalog No.:BCC7309
CAS No.:113190-92-4
- H-Phe(4-F)-OH
Catalog No.:BCC3216
CAS No.:1132-68-9
- (3R)-Hydrangenol 8-O-glucoside pentaacetate
Catalog No.:BCN1617
CAS No.:113270-98-7
- (3S)-Hydrangenol 8-O-glucoside pentaacetate
Catalog No.:BCN1616
CAS No.:113270-99-8
- ABT-333
Catalog No.:BCC4129
CAS No.:1132935-63-7
- GDC-0834
Catalog No.:BCC5115
CAS No.:1133432-50-4
- neo-Truxilline
Catalog No.:BCN1949
CAS No.:113350-54-2
- γ-Truxilline
Catalog No.:BCN1948
CAS No.:113350-56-4
- 2-Amino-4-phenylphenol
Catalog No.:BCC8534
CAS No.:1134-36-7
- Baclofen
Catalog No.:BCC8839
CAS No.:1134-47-0
- 2-Aminophenyl phenyl sulfide
Catalog No.:BCC8553
CAS No.:1134-94-7
Perivascular expression and potent vasoconstrictor effect of dynorphin A in cerebral arteries.[Pubmed:22662226]
PLoS One. 2012;7(5):e37798.
BACKGROUND: Numerous literary data indicate that dynorphin A (DYN-A) has a significant impact on cerebral circulation, especially under pathophysiological conditions, but its potential direct influence on the tone of cerebral vessels is obscure. The aim of the present study was threefold: 1) to clarify if DYN-A is present in cerebral vessels, 2) to determine if it exerts any direct effect on cerebrovascular tone, and if so, 3) to analyze the role of kappa-opiate receptors in mediating the effect. METHODOLOGY/PRINCIPAL FINDINGS: Immunohistochemical analysis revealed the expression of DYN-A in perivascular nerves of rat pial arteries as well as in both rat and human intraparenchymal vessels of the cerebral cortex. In isolated rat basilar and middle cerebral arteries (BAs and MCAs) DYN-A (1-13) and DYN-A (1-17) but not DYN-A (1-8) or dynorphin B (DYN-B) induced strong vasoconstriction in micromolar concentrations. The maximal effects, compared to a reference contraction induced by 124 mM K(+), were 115+/-6% and 104+/-10% in BAs and 113+/-3% and 125+/-9% in MCAs for 10 microM of DYN-A (1-13) and DYN-A (1-17), respectively. The vasoconstrictor effects of DYN-A (1-13) could be inhibited but not abolished by both the kappa-opiate receptor antagonist nor-Binaltorphimine dihydrochloride (NORBI) and blockade of G(i/o)-protein mediated signaling by pertussis toxin. Finally, des-Tyr(1) DYN-A (2-13), which reportedly fails to activate kappa-opiate receptors, induced vasoconstriction of 45+/-11% in BAs and 50+/-5% in MCAs at 10 microM, which effects were resistant to NORBI. CONCLUSION/SIGNIFICANCE: DYN-A is present in rat and human cerebral perivascular nerves and induces sustained contraction of rat cerebral arteries. This vasoconstrictor effect is only partly mediated by kappa-opiate receptors and heterotrimeric G(i/o)-proteins. To our knowledge our present findings are the first to indicate that DYN-A has a direct cerebral vasoconstrictor effect and that a dynorphin-induced vascular action may be, at least in part, independent of kappa-opiate receptors.
Differential agonist regulation of the human kappa-opioid receptor.[Pubmed:9109509]
J Neurochem. 1997 May;68(5):1846-52.
Opiates are potent analgesics used clinically in the treatment of pain. A significant drawback to the chronic use and clinical effectiveness of opiates is the development of tolerance. To investigate the cellular mechanisms of tolerance, the cloned human kappa-opioid receptor was stably expressed in human embryonic kidney (HEK 293) cells, and the effects of opioid agonist treatment were examined. The receptor-expressing cells showed specific high-affinity membrane binding for a kappa-selective opioid, 3H-labeled (+)-(5alpha,7alpha,8beta)-N-methyl-N-[7-(1-pyrrolidiny l)-1-oxaspiro [4,5] dec-8-yl] benzeneacetamide ([3H]U69,593), and a nonselective opioid antagonist, [3H]diprenorphine. Pretreatment with pertussis toxin or guanosine 5'-O-(3-thiotriphosphate) reduced [3H]69,593 binding, indicating that the human K receptor coupled to G proteins of the Gi or Go families in HEK 293 cells. The receptor-mediated inhibition of adenylyl cyclase was abolished by pertussis toxin pretreatment and was blocked by a kappa-selective antagonist, norbinaltorphimine. A 3-h pretreatment with a kappa-selective agonist, (+/-)-trans-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl] benzeneacetamide (U50,488), caused receptor down-regulation, whereas no receptor down-regulation was found after levorphanol pretreatment. U50,488 or dynorphin A(1-17) pretreatments (3 h) desensitized the ability of U50,488 or dynorphin A(1-17) to inhibit cyclic AMP accumulation, as evidenced by a decrease in functional potency. Also, U50,488 pretreatment desensitized the ability of levorphanol to inhibit forskolin-stimulated cyclic AMP accumulation. In contrast, pretreatment of cells with either levorphanol or a potent nonselective opioid, etorphine, resulted in no apparent receptor desensitization. Taken together, these results demonstrate that the human kappa receptor is differentially regulated by selective and nonselective opioid agonists, with selective agonists able to desensitize the receptor.
Nor-binaltorphimine precipitates withdrawal and excitatory amino acid release in the locus ceruleus of butorphanol--but not morphine-dependent rats.[Pubmed:9353416]
J Pharmacol Exp Ther. 1997 Nov;283(2):932-8.
The relative involvement of kappa opioid receptors in the mediation of behavioral and neurochemical responses to withdrawal from chronic drug treatment with the opioid analgesic butorphanol was studied using in vivo microdialysis to detail extracellular fluid concentrations of glutamate and aspartate within the locus ceruleus. Sprague-Dawley rats were rendered opioid dependent after 3 days of intracerebroventricular (i.c.v.) infusion of butorphanol (26 nmol/microl/hr) or morphine (26 nmol/microl/hr) and after i.c.v. infusion of saline vehicle (1 microl/hr). Acute withdrawal was precipitated by i.c.v. injection of the selective kappa opioid receptor antagonist nor-binaltorphimine (48 nmol/5 microl) after the 3-day period of infusion. Behavioral signs of withdrawal were detected after nor-binaltorphimine only in butorphanol-dependent rats. Basal levels of glutamate and aspartate were not different between treatment groups. Nor-binaltorphimine in the butorphanol-dependent rats increased glutamate to 227% and aspartate to 158% in the initial 15-min sample (P < 0.01). Nor-binaltorphimine did not increase glutamate or aspartate concentrations in the morphine-dependent or saline-treated groups. These results indicate a significantly greater participation of kappa opioid receptors in the development of butorphanol, rather than morphine, dependence and identify a differential neurochemical response to butorphanol withdrawal within a defined brain region, the locus ceruleus.
Structure-activity relationship of N17'-substituted norbinaltorphimine congeners. Role of the N17' basic group in the interaction with a putative address subsite on the kappa opioid receptor.[Pubmed:8182708]
J Med Chem. 1994 May 13;37(10):1495-500.
A series of norbinaltorphimine congeners (2-12) which contain different groups at the N17'-position have been synthesized in order to evaluate the role of N17' in conferring kappa opioid antagonist selectivity at opioid receptor sites. The compounds that contain a basic N17' nitrogen (2-9) were found to be selective kappa antagonists. Amidation of N17' afforded congeners 10-12 with feeble kappa antagonist potency and low selectivity. The fact that potent antagonism and selectivity were observed only when members of the series contain a basic N17' nitrogen suggests that it interacts with extracellular domains of the kappa receptor that contain acidic amino acid residues. The N-terminal domain and extracellular loop 2, both of which contain acidic residues, are candidates for this interaction and may be components of the kappa address subsite of the receptor.


