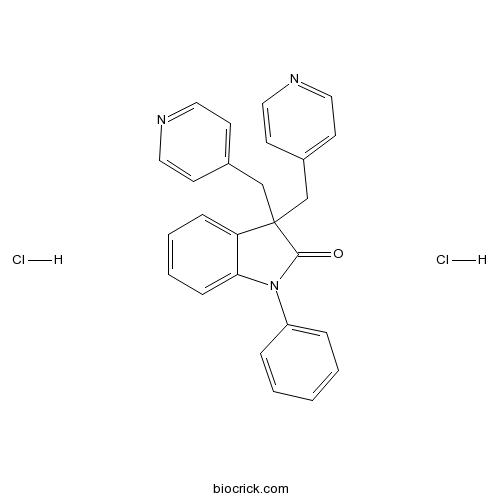
Linopirdine dihydrochlorideKV7 (KCNQ) channel blocker CAS# 113168-57-3 |
- SKLB610
Catalog No.:BCC3647
CAS No.:1125780-41-7
- Axitinib (AG 013736)
Catalog No.:BCC3729
CAS No.:319460-85-0
- Vandetanib (ZD6474)
Catalog No.:BCC3883
CAS No.:443913-73-3
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- SU14813 double bond Z
Catalog No.:BCC1972
CAS No.:452105-23-6
- Brivanib Alaninate (BMS-582664)
Catalog No.:BCC1240
CAS No.:649735-63-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 113168-57-3 | SDF | Download SDF |
| PubChem ID | 14209557 | Appearance | Powder |
| Formula | C26H23Cl2N3O | M.Wt | 464.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DuP 996 | ||
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
| Chemical Name | 1-phenyl-3,3-bis(pyridin-4-ylmethyl)indol-2-one;dihydrochloride | ||
| SMILES | C1=CC=C(C=C1)N2C3=CC=CC=C3C(C2=O)(CC4=CC=NC=C4)CC5=CC=NC=C5.Cl.Cl | ||
| Standard InChIKey | ZEVVHCGTTNRYOY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H21N3O.2ClH/c30-25-26(18-20-10-14-27-15-11-20,19-21-12-16-28-17-13-21)23-8-4-5-9-24(23)29(25)22-6-2-1-3-7-22;;/h1-17H,18-19H2;2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Blocker of KV7 (KCNQ) voltage-gated potassium channels; blocks KV7.1+7.3 (KCNQ2+3) / M-currents (IC50 = 4 - 7 μM) and KV7.1 (KCNQ1) homomeric channels (IC50 = 8.9 μM). Augments hippocampal ACh release and is a cognitive enhancer following oral administration in vivo. |

Linopirdine dihydrochloride Dilution Calculator

Linopirdine dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1534 mL | 10.7668 mL | 21.5336 mL | 43.0672 mL | 53.8341 mL |
| 5 mM | 0.4307 mL | 2.1534 mL | 4.3067 mL | 8.6134 mL | 10.7668 mL |
| 10 mM | 0.2153 mL | 1.0767 mL | 2.1534 mL | 4.3067 mL | 5.3834 mL |
| 50 mM | 0.0431 mL | 0.2153 mL | 0.4307 mL | 0.8613 mL | 1.0767 mL |
| 100 mM | 0.0215 mL | 0.1077 mL | 0.2153 mL | 0.4307 mL | 0.5383 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- nor-Binaltorphimine dihydrochloride
Catalog No.:BCC6614
CAS No.:113158-34-2
- Physalin L
Catalog No.:BCN2312
CAS No.:113146-74-0
- (S)-(+)-Niguldipine hydrochloride
Catalog No.:BCC6947
CAS No.:113145-69-0
- 3-Deoxysappanone B
Catalog No.:BCN6012
CAS No.:113122-54-6
- 7-O-Methylrosmanol
Catalog No.:BCN7276
CAS No.:113085-62-4
- VU 0155069
Catalog No.:BCC7715
CAS No.:1130067-06-9
- Potassium benzylpenicillin
Catalog No.:BCC9126
CAS No.:113-98-4
- Chlorpheniramine Maleate
Catalog No.:BCC4526
CAS No.:113-92-8
- Chlorprothixene
Catalog No.:BCC3753
CAS No.:113-59-7
- Estradiol diproppionate
Catalog No.:BCC8960
CAS No.:113-38-2
- IM-12
Catalog No.:BCC5487
CAS No.:1129669-05-1
- Tokinolide B
Catalog No.:BCN2753
CAS No.:112966-16-2
- PPPA
Catalog No.:BCC7309
CAS No.:113190-92-4
- H-Phe(4-F)-OH
Catalog No.:BCC3216
CAS No.:1132-68-9
- (3R)-Hydrangenol 8-O-glucoside pentaacetate
Catalog No.:BCN1617
CAS No.:113270-98-7
- (3S)-Hydrangenol 8-O-glucoside pentaacetate
Catalog No.:BCN1616
CAS No.:113270-99-8
- ABT-333
Catalog No.:BCC4129
CAS No.:1132935-63-7
- GDC-0834
Catalog No.:BCC5115
CAS No.:1133432-50-4
- neo-Truxilline
Catalog No.:BCN1949
CAS No.:113350-54-2
- γ-Truxilline
Catalog No.:BCN1948
CAS No.:113350-56-4
- 2-Amino-4-phenylphenol
Catalog No.:BCC8534
CAS No.:1134-36-7
- Baclofen
Catalog No.:BCC8839
CAS No.:1134-47-0
- 2-Aminophenyl phenyl sulfide
Catalog No.:BCC8553
CAS No.:1134-94-7
- Jatamanvaltrate B
Catalog No.:BCN7128
CAS No.:1134138-66-1
Selectivity of linopirdine (DuP 996), a neurotransmitter release enhancer, in blocking voltage-dependent and calcium-activated potassium currents in hippocampal neurons.[Pubmed:9694925]
J Pharmacol Exp Ther. 1998 Aug;286(2):709-17.
Linopirdine [DuP 996, 3, 3-bis(4-pyridinylmethyl)-1-phenylindolin-2-one], a putative cognition enhancing drug, increases acetylcholine release in rat brain tissue and improves performance in animal models of learning and memory. The mechanism whereby linopirdine enhances acetylcholine release has been proposed to involve inhibition of the M-type K+ current (IM). Our study examines the selectivity of linopirdine for IM by determining its effects on other ionic currents present in rat hippocampal CA1 neurons using patch clamp techniques. Linopirdine was found to block voltage-gated, calcium-activated and leak K+ currents in a dose-dependent manner. Of the seven currents measured, linopirdine was most selective for IM with an IC50 of 2.4 +/- 0.4 microM, followed by IC (measured as a medium afterhyperpolarization tail current, ImAHP) with an IC50 of 16.3 +/- 2.4 microM. Both IM and IC were completely suppressed by linopirdine. At a concentration of 100 microM, linopirdine weakly inhibited the K+ leak current, IL, the transient outward current, IA, the delayed rectifier, IK, and the slow component of IAHP, by 28 +/- 8, 37 +/- 10, 36 +/- 9 and 52 +/- 10 percent, respectively. The mixed Na+/K+ inward rectifying current, IQ, was essentially unaffected by linopirdine (IC50 >300 microM). These results indicate that linopirdine selectively blocks IM at concentrations
KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel.[Pubmed:9836639]
Science. 1998 Dec 4;282(5395):1890-3.
The M-current regulates the subthreshold electrical excitability of many neurons, determining their firing properties and responsiveness to synaptic input. To date, however, the genes that encode subunits of this important channel have not been identified. The biophysical properties, sensitivity to pharmacological blockade, and expression pattern of the KCNQ2 and KCNQ3 potassium channels were determined. It is concluded that both these subunits contribute to the native M-current.
Two new potent neurotransmitter release enhancers, 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone and 10,10-bis(2-fluoro-4-pyridinylmethyl)-9(10H)-anthracenone: comparison to linopirdine.[Pubmed:9580619]
J Pharmacol Exp Ther. 1998 May;285(2):724-30.
Linopirdine (3,3-bis(4-pyridinylmethyl)-1-phenylindolin-2-one, DUP996) is an extensively studied representative of a class of cognition enhancing compounds that increase the evoked release of neurotransmitters. Recent studies suggest that these agents act through the blockade of specific K+ channels. We have recently identified more potent anthracenone analogs of linopirdine: 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone (XE991) and 10,10-bis(2-fluoro-4-pyridinylmethyl)-9(10H)-anthracenone (DMP 543). Although linopirdine possesses an EC50 of 4.2 microM for enhancement of [3H]ACh release from rat brain slices, XE991 and DMP 543 have EC50S of 490 and 700 nM, respectively. In addition to greater in vitro potency relative to linopirdine, both compounds show greater in vivo potency and duration of action. Although 5 mg/kg (p.o.) linopirdine does not lead to statistically significant increases in hippocampal extracellular acetylcholine levels, 5 mg/kg (p.o.) XE991 leads to increases (maximal effect > 90% over baseline) which are sustained for 60 min. Moreover, DMP 543 at 1 mg/kg causes more than a 100% increase in acetylcholine levels with the effect lasting more than 3 hr. At doses relevant to their release-enhancing properties, the only overt symptom consistently observed was tremor, possible via a cholinergic mechanism. These results suggest that XE991 and DMP 543 may prove to be superior to linopirdine as Alzheimer's disease therapeutics. In addition, these agents should be useful pharmacological tools for probing the importance of particular ion channels in the control of neurotransmitter release.


