Ferulic acidCAS# 1135-24-6 |
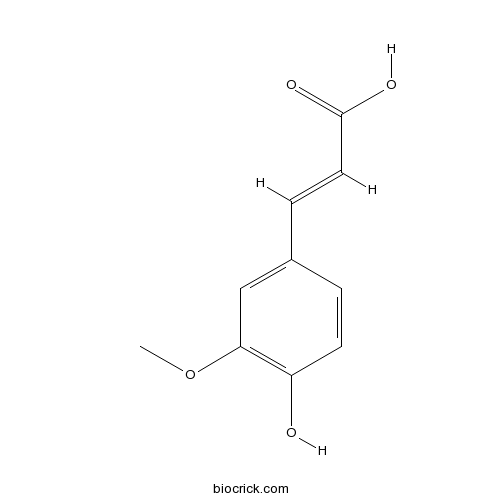
- trans-Ferulic acid
Catalog No.:BCN0322
CAS No.:537-98-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1135-24-6 | SDF | Download SDF |
| PubChem ID | 445858 | Appearance | Powder |
| Formula | C10H10O4 | M.Wt | 194.19 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | Trans-Ferulic acid;537-98-4;97274-61-8 | ||
| Solubility | DMSO : 100 mg/mL (514.99 mM; Need ultrasonic) | ||
| Chemical Name | (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid | ||
| SMILES | COC1=C(C=CC(=C1)C=CC(=O)O)O | ||
| Standard InChIKey | KSEBMYQBYZTDHS-HWKANZROSA-N | ||
| Standard InChI | InChI=1S/C10H10O4/c1-14-9-6-7(2-4-8(9)11)3-5-10(12)13/h2-6,11H,1H3,(H,12,13)/b5-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ferulic acid is an antioxidant found naturally in plant cell walls , shows antioxidant activity in parallel with their radical scavenging activity, it has been approved in certain countries as food additive to prevent lipid peroxidation. Ferulic acid has been reported to have many physiological functions, including antioxidant, antimicrobial, anti-inflammatory, anti-thrombosis, anti- diabetic, and anti-cancer activities; it also protects against coronary disease, lowers cholesterol and increases sperm viability.Trans-Ferulic acid has antioxidant activity, it dose-dependently reduces lipid peroxidation induced by the three oxidants; it exerts a protective action on liver injury induced by chronic ethanol ingestion. |
| Targets | ROS | SOD | GPx | CAT | Antifection | NADPH-oxidase |
| In vitro | Ferulic acid: pharmaceutical functions, preparation and applications in foods.[Reference: WebLink]J. Sci. Food Agr., 2004, 84(11):1261-9.
Ferulic acid (4‐hydroxy‐3‐methoxycinnamic acid), an effective component of Chinese medicine herbs such as Angelica sinensis, Cimicifuga heracleifolia and Lignsticum chuangxiong, is a ubiquitous phenolic acid in the plant kingdom. It is mainly conjugated with mono‐ and oligosaccharides, polyamines, lipids and polysaccharides and seldom occurs in a free state in plants. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes.[Pubmed: 23668982]Colloids Surf B Biointerfaces. 2013 Sep 1;109:273-9.
L-lysine pro-prodrug containing trans-ferulic acid for 5-amino salicylic acid colon delivery: synthesis, characterization and in vitro antioxidant activity evaluation.[Pubmed: 20045975]Chem Pharm Bull (Tokyo). 2010 Jan;58(1):103-5.In the present work, we report the synthesis of a new 5-amino salicylic acid (5-ASA) pro-prodrug, useful in Crohn disease treatment, and the evaluation of its antioxidant activity. |
| In vivo | Ferulic acid alleviates lipid peroxidation in diabetic rats.[Pubmed: 15162367 ]Phytother Res. 2004 Apr;18(4):310-4.Diabetes mellitus is a metabolic disorder associated with increased formation of free radicals. The objective of our study was to determine whether Ferulic acid (FA), a phenolic acid, has any role to play in diabetes induced free radical formation. The effect of trans-ferulic acid and gamma-oryzanol on ethanol-induced liver injury in C57BL mouse[Pubmed: 18424018]Phytomedicine. 2008 Nov;15(11):951-8.The effects of the oral administration of Trans-Ferulic acid and gamma-oryzanol (mixture of steryl ferulates) with ethanol (5.0 g per kg) for 30 days to c57BL mice on ethanol-induced liver injury were investigated. |
| Kinase Assay | A potent inhibition of oxidative stress induced gene expression in neural cells by sustained ferulic acid release from chitosan based hydrogel.[Pubmed: 25686998]Mater Sci Eng C Mater Biol Appl. 2015 Apr;49:691-9.Ferulic acid (4‐hydroxy‐3‐methoxycinnamic acid), an effective component of Chinese medicine herbs such as Angelica sinensis, Cimicifuga heracleifolia and Lignsticum chuangxiong, is a ubiquitous phenolic acid in the plant kingdom. |
| Structure Identification | Crit Rev Biotechnol. 2004;24(2-3):59-83.Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications.[Pubmed: 15493526 ]Ferulic acid is the most abundant hydroxycinnamic acid in the plant world and maize bran with 3.1% (w/w) Ferulic acid is one of the most promising sources of this antioxidant. |

Ferulic acid Dilution Calculator

Ferulic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.1496 mL | 25.748 mL | 51.496 mL | 102.9919 mL | 128.7399 mL |
| 5 mM | 1.0299 mL | 5.1496 mL | 10.2992 mL | 20.5984 mL | 25.748 mL |
| 10 mM | 0.515 mL | 2.5748 mL | 5.1496 mL | 10.2992 mL | 12.874 mL |
| 50 mM | 0.103 mL | 0.515 mL | 1.0299 mL | 2.0598 mL | 2.5748 mL |
| 100 mM | 0.0515 mL | 0.2575 mL | 0.515 mL | 1.0299 mL | 1.2874 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ivacaftor benzenesulfonate
Catalog No.:BCC1663
CAS No.:1134822-09-5
- Ivacaftor hydrate
Catalog No.:BCC1664
CAS No.:1134822-07-3
- Epidanshenspiroketallactone
Catalog No.:BCN3142
CAS No.:113472-19-8
- Rhein-8-glucoside calcium salt
Catalog No.:BCN6349
CAS No.:113443-70-2
- VER 155008
Catalog No.:BCC2338
CAS No.:1134156-31-2
- Jatamanvaltrate B
Catalog No.:BCN7128
CAS No.:1134138-66-1
- 2-Aminophenyl phenyl sulfide
Catalog No.:BCC8553
CAS No.:1134-94-7
- Baclofen
Catalog No.:BCC8839
CAS No.:1134-47-0
- 2-Amino-4-phenylphenol
Catalog No.:BCC8534
CAS No.:1134-36-7
- γ-Truxilline
Catalog No.:BCN1948
CAS No.:113350-56-4
- neo-Truxilline
Catalog No.:BCN1949
CAS No.:113350-54-2
- GDC-0834
Catalog No.:BCC5115
CAS No.:1133432-50-4
- Moxidectin
Catalog No.:BCC5309
CAS No.:113507-06-5
- Tracazolate hydrochloride
Catalog No.:BCC7115
CAS No.:1135210-68-2
- VU 0357017 hydrochloride
Catalog No.:BCC7907
CAS No.:1135242-13-5
- VU 0255035
Catalog No.:BCC7766
CAS No.:1135243-19-4
- SR 59230A hydrochloride
Catalog No.:BCC7094
CAS No.:1135278-41-9
- CGP 78608 hydrochloride
Catalog No.:BCC7087
CAS No.:1135278-54-4
- 3'-Fluorobenzylspiperone maleate
Catalog No.:BCC6752
CAS No.:1135278-61-3
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- Altanserin hydrochloride
Catalog No.:BCC7183
CAS No.:1135280-78-2
- 6-Bnz-cAMP sodium salt
Catalog No.:BCC8043
CAS No.:1135306-29-4
- 25(S)-Hydroxyprotopanaxatriol
Catalog No.:BCN2495
CAS No.:113539-03-0
- Ac-IEPD-AFC
Catalog No.:BCC2358
CAS No.:1135417-31-0
Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications.[Pubmed:15493526]
Crit Rev Biotechnol. 2004;24(2-3):59-83.
Ferulic acid is the most abundant hydroxycinnamic acid in the plant world and maize bran with 3.1% (w/w) Ferulic acid is one of the most promising sources of this antioxidant. The dehydrodimers of Ferulic acid are important structural components in the plant cell wall and serve to enhance its rigidity and strength. Feruloyl esterases are a subclass of the carboxylic acid esterases that hydrolyze the ester bond between hydroxycinnamic acids and sugars present in plant cell walls and they have been isolated from a wide range of microorganisms, when grown on complex substrates such as cereal brans, sugar beet pulp, pectin and xylan. These enzymes perform a function similar to alkali in the deesterification of plant cell wall and differ in their specificities towards the methyl esters of cinnamic acids and ferulolylated oligosaccharides. They act synergistically with xylanases and pectinases and facilitate the access of hydrolases to the backbone of cell wall polymers. The applications of Ferulic acid and feruloyl esterase enzymes are many and varied. Ferulic acid obtained from agricultural byproducts is a potential precursor for the production of natural vanillin, due to the lower production cost.
Ferulic acid alleviates lipid peroxidation in diabetic rats.[Pubmed:15162367]
Phytother Res. 2004 Apr;18(4):310-4.
Diabetes mellitus is a metabolic disorder associated with increased formation of free radicals. The objective of our study was to determine whether Ferulic acid (FA), a phenolic acid, has any role to play in diabetes induced free radical formation. Diabetes was induced with streptozotocin. The levels of blood glucose, thiobarbituric acid reactive substances (TBARS), hydroperoxides and free fatty acids (FFA) increased in the liver of diabetic animals. The activities of glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT) decreased in the liver. Histopathology of pancreas also shows shrunken islets. Supplementation of FA to the diabetic rats resulted in a decrease in the levels of glucose, TBARS, hydroperoxides, FFA and an increase in reduced glutathione (GSH). FA also resulted in increased activities of SOD, CAT, GPx and expansion of pancreatic islets. The effect was much pronounced with lower dose treatment. Thus our study shows that administration of Ferulic acid helps in enhancing the antioxidant capacity of these diabetic animals by neutralizing the free radicals formed thereby reducing the intensity of diabetes.
A new pro-prodrug aminoacid-based for trans-ferulic Acid and silybin intestinal release.[Pubmed:25062426]
J Funct Biomater. 2014 Jul 24;5(3):99-110.
The aim of this work was the preparation and characterization of a pro-prodrug able to simultaneously transport silybin, a drug possessing various pharmacological effects, and trans-Ferulic acid, a known antioxidant. More specifically, l-phenylalanine-N-(4-hydroxy-3-methoxy-phenyl) prop-2-en-O-(2R,3R)-3,5,7-trihydroxy-2-((2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-2-( hydroxymethyl)-2,3-dihydro-benzo-(1,4)-dioxin-6-yl)croman-4-one was synthesized by using the aminoacid l-phenylalanine (l-Phe) as carrier. Indeed, l-Phe is characterized by an intrinsic chemical reactivity due to the presence of an amino group, placed on the chiral center, and of a carboxylic group. The synthesis has been characterized first by adding silybin by means of carboxylic group and then, with the aim to confer antioxidant properties to this new carrier, by linking trans-Ferulic acid to l-Phe via amino group. The so obtained derivative was then characterized by FT-IR, and 1H-NMR spectroscopies. Furthermore, its ability to inhibit lipid peroxidation induced by tert-butyl hydroperoxide in rat liver microsomes, was evaluated. The 1,1-diphenyl-2-picrylhydrazyl radical-scavenging effect, was also assessed. The release of silybin and trans-Ferulic acid was determined in simulated gastric and intestinal fluids over the time. The results showed that the covalent bond between both (i) silybin; or (ii) trans-Ferulic acid and the amino acid was degraded by enzymatic reactions. In addition, the pro-prodrug, showed strong antioxidant and scavenger activities. Due to these properties, this new pro-prodrug could be applied for the treatment of intestinal pathologies and it might improve the therapeutic potential of silybin which is strongly limited by its low solubility.
L-lysine pro-prodrug containing trans-ferulic acid for 5-amino salicylic acid colon delivery: synthesis, characterization and in vitro antioxidant activity evaluation.[Pubmed:20045975]
Chem Pharm Bull (Tokyo). 2010 Jan;58(1):103-5.
In the present work, we report the synthesis of a new 5-amino salicylic acid (5-ASA) pro-prodrug, useful in Crohn disease treatment, and the evaluation of its antioxidant activity. Using as pharmacological carrier L-lysine amino acid and taking advantage of its intrinsic chemical reactivity, due to the presence of two amino groups, placed on the chiral center and in epsilon-position, we inserted trans-Ferulic acid in epsilon-position, through amidation reaction, esterified with methanol the carboxylic group and, finally, submitted the free amino group to diazotation with 5-ASA, principal drug for inflammatory bowel diseases (IBD) care. All intermediates of synthesis and the final product (derivative A) were characterized with usual spectroscopic techniques, as FT-IR, GC/MS and (1)H-MNR. Finally, the derivative A antioxidant activity in inhibiting the lipid peroxidation, in rat-liver microsomal membranes, induced in vitro by two different sources of free radicals, 2,2'-azobis (2-amidinopropane) (AAPH) and tert-butyl hydroperoxide (tert-BOOH), was evaluated. Our pro-prodrug could be successfully applied in pharmaceutical field both as prodrug of 5-ASA than as carrier of trans-Ferulic acid.
The effect of trans-ferulic acid and gamma-oryzanol on ethanol-induced liver injury in C57BL mouse.[Pubmed:18424018]
Phytomedicine. 2008 Nov;15(11):951-8.
The effects of the oral administration of trans-Ferulic acid and gamma-oryzanol (mixture of steryl ferulates) with ethanol (5.0 g per kg) for 30 days to c57BL mice on ethanol-induced liver injury were investigated. Preventions of ethanol-induced liver injury by trans-Ferulic acid and gamma-oryzanol were reflected by markedly decreased serum activities of plasma aspartate aminotransferase, alanine aminotransferase and significant decreases in hepatic lipid hydroperoxide and TBARS levels. Furthermore, the trans-Ferulic acid- and gamma-oryzanol-treated mice recovered ethanol-induced decrease in hepatic glutathione level together with enhancing superoxide dismutase activity. These results demonstrate that both trans-Ferulic acid and gamma-oryzanol exert a protective action on liver injury induced by chronic ethanol ingestion.
A potent inhibition of oxidative stress induced gene expression in neural cells by sustained ferulic acid release from chitosan based hydrogel.[Pubmed:25686998]
Mater Sci Eng C Mater Biol Appl. 2015 Apr;49:691-699.
Traumatic brain injury (TBI) is an extremely cataclysmic neurological disorder and the inhibition of oxidative stress following TBI could effectively protect the brain from further impairments. An injectable thermosensitive chitosan/gelatin/beta-Glycerol phosphate (C/G/GP) hydrogel for the controlled release of the phenolic antioxidant Ferulic acid (FA) to inhibit the neurological oxidative stress was demonstrated. The C/G/GP hydrogel ensures an excellent clinical expediency with a gelation temperature of 32.6 degrees C and gelation time of 75.58s. In-vitro cytotoxicity assays of C/G/GP hydrogel and FA have revealed an excellent biocompatibility with the Neuro-2a cells. 500muM of FA was considered to be an effective concentration to reduce the oxidative stress in Neuro-2a cells. TUNEL staining images evidenced that the H2O2 induced DNA fragmentation was comprehensively controlled after FA treatment. The mRNA gene expression profiles markedly authenticate the neuroprotectivity of FA by down-regulating ROS, inflammatory and apoptosis related markers. The outcomes of this study suggest that, C/G/GP hydrogel carrying Ferulic acid could effectively protect further secondary traumatic brain injury associated impairments.
Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes.[Pubmed:23668982]
Colloids Surf B Biointerfaces. 2013 Sep 1;109:273-9.
In this study, stearic acid- and stearyl ferulate-based solid lipid nanoparticles containing trans-Ferulic acid (SLN-FA and SLN-SF-FA, respectively), were prepared and characterized for loading efficiency, size and shape. In addition, by using rat brain microsomes, we evaluated in vitro the antioxidant activity of these formulations against three well known initiators of lipid peroxidation, such as AAPH, NADPH/ADP-Fe(3+) and SIN-1 which in turn generate the peroxyl and perferryl radicals as well as peroxynitrite, respectively. Commercially available FA and its ethyl ester (FAEE) were used as comparators. Both SLN-FA and SLN-SF-FA dose-dependently reduced lipid peroxidation induced by the three oxidants. Interestingly, SLN-SF-FA displayed greater efficacy (EC50) and potency (maximal activity) against AAPH- and NADPH/ADP-Fe(3+)-induced lipid peroxidation. Our results support the idea that this new formulations could facilitate the uptake of FA by the cells because of their lipophilic structure, thus increasing FA bioavailability. Furthermore, stearyl ferulate-based nanoparticles could prevent the degradation of FA entrapped on their structure, making FA almost entirely available to explicate its antioxidant power once released.


