Tracazolate hydrochlorideSubtype-selective GABAA allosteric modulator CAS# 1135210-68-2 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1135210-68-2 | SDF | Download SDF |
| PubChem ID | 24871267 | Appearance | Powder |
| Formula | C16H25ClN4O2 | M.Wt | 340.85 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ICI 136753 | ||
| Solubility | Soluble to 100 mM in 1eq. HCl | ||
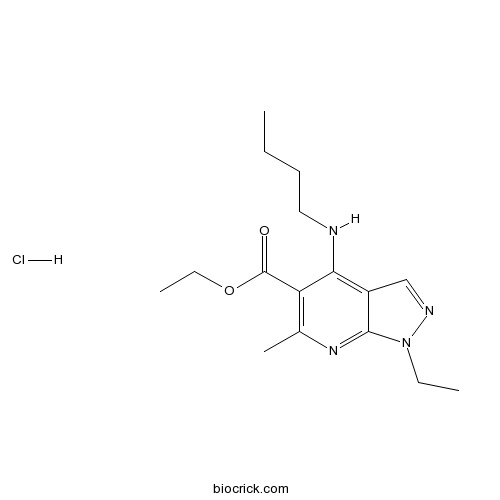
| Chemical Name | ethyl 4-(butylamino)-1-ethyl-6-methylpyrazolo[3,4-b]pyridine-5-carboxylate;hydrochloride | ||
| SMILES | CCCCNC1=C2C=NN(C2=NC(=C1C(=O)OCC)C)CC.Cl | ||
| Standard InChIKey | TUIXDPUEMQXVHO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H24N4O2.ClH/c1-5-8-9-17-14-12-10-18-20(6-2)15(12)19-11(4)13(14)16(21)22-7-3;/h10H,5-9H2,1-4H3,(H,17,19);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Subtype-selective GABAA receptor modulator. Can potentiate or inhibit recombinant GABAA function depending on subunit combination. Enhances native GABAA receptor function and is anxiolytic following systemic administration in vivo. |

Tracazolate hydrochloride Dilution Calculator

Tracazolate hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9338 mL | 14.6692 mL | 29.3384 mL | 58.6768 mL | 73.346 mL |
| 5 mM | 0.5868 mL | 2.9338 mL | 5.8677 mL | 11.7354 mL | 14.6692 mL |
| 10 mM | 0.2934 mL | 1.4669 mL | 2.9338 mL | 5.8677 mL | 7.3346 mL |
| 50 mM | 0.0587 mL | 0.2934 mL | 0.5868 mL | 1.1735 mL | 1.4669 mL |
| 100 mM | 0.0293 mL | 0.1467 mL | 0.2934 mL | 0.5868 mL | 0.7335 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Moxidectin
Catalog No.:BCC5309
CAS No.:113507-06-5
- Ferulic acid
Catalog No.:BCN5948
CAS No.:1135-24-6
- Ivacaftor benzenesulfonate
Catalog No.:BCC1663
CAS No.:1134822-09-5
- Ivacaftor hydrate
Catalog No.:BCC1664
CAS No.:1134822-07-3
- Epidanshenspiroketallactone
Catalog No.:BCN3142
CAS No.:113472-19-8
- Rhein-8-glucoside calcium salt
Catalog No.:BCN6349
CAS No.:113443-70-2
- VER 155008
Catalog No.:BCC2338
CAS No.:1134156-31-2
- Jatamanvaltrate B
Catalog No.:BCN7128
CAS No.:1134138-66-1
- 2-Aminophenyl phenyl sulfide
Catalog No.:BCC8553
CAS No.:1134-94-7
- Baclofen
Catalog No.:BCC8839
CAS No.:1134-47-0
- 2-Amino-4-phenylphenol
Catalog No.:BCC8534
CAS No.:1134-36-7
- γ-Truxilline
Catalog No.:BCN1948
CAS No.:113350-56-4
- VU 0357017 hydrochloride
Catalog No.:BCC7907
CAS No.:1135242-13-5
- VU 0255035
Catalog No.:BCC7766
CAS No.:1135243-19-4
- SR 59230A hydrochloride
Catalog No.:BCC7094
CAS No.:1135278-41-9
- CGP 78608 hydrochloride
Catalog No.:BCC7087
CAS No.:1135278-54-4
- 3'-Fluorobenzylspiperone maleate
Catalog No.:BCC6752
CAS No.:1135278-61-3
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- Altanserin hydrochloride
Catalog No.:BCC7183
CAS No.:1135280-78-2
- 6-Bnz-cAMP sodium salt
Catalog No.:BCC8043
CAS No.:1135306-29-4
- 25(S)-Hydroxyprotopanaxatriol
Catalog No.:BCN2495
CAS No.:113539-03-0
- Ac-IEPD-AFC
Catalog No.:BCC2358
CAS No.:1135417-31-0
- Magnoloside A
Catalog No.:BCN6013
CAS No.:113557-95-2
- 1,2,3,19-Tetrahydroxy-12-ursen-28-oic acid
Catalog No.:BCN1615
CAS No.:113558-03-5
Tracazolate reveals a novel type of allosteric interaction with recombinant gamma-aminobutyric acid(A) receptors.[Pubmed:11901225]
Mol Pharmacol. 2002 Apr;61(4):861-9.
Tracazolate, a pyrazolopyridine, is an anxiolytic known to interact with gamma-aminobutyric acid (GABA)(A) receptors, adenosine receptors, and phosphodiesterases. Its anxiolytic effect is thought to be via its interaction with GABA(A) receptors. We now report the first detailed pharmacological study examining the effects of tracazolate on a range of recombinant GABA(A) receptors expressed in Xenopus laevis oocytes. Replacement of the gamma2s subunit within the alpha1beta3gamma2s receptor with the epsilon subunit caused a dramatic change in the functional response to tracazolate from potentiation to inhibition. The gamma2s subunit was not critical for potentiation because alpha1beta3 receptors were also potentiated by tracazolate. gamma2/epsilon chimeras revealed a critical N-terminal domain between amino acids 206 and 230 of gamma2, governing the nature of this response. Replacement of the beta3 subunit with the beta1 subunit within alpha1beta3gamma2s and alpha1beta3epsilon receptors also revealed selectivity of tracazolate for beta3-containing receptors, determined by asparagine at position 265 within transmembrane 2. Replacement of gamma2s with gamma1 or gamma3 revealed a profile intermediate to that of alpha1beta1epsilon and alpha1beta1gamma2s. alpha1beta1delta receptors were also potentiated by tracazolate; however, the maximum potentiation of the EC(20) was much greater than on alpha1beta1gamma2. Concentration-response curves to GABA in the presence of tracazolate for alpha1beta1epsilon and alpha1beta1gamma2s revealed a concentration-related decrease in maximum current amplitude, but a leftward shift in the EC(50) only on alpha1beta1gamma2. Like alpha1beta1gamma2s, GABA concentration-response curves on alpha1beta1delta receptors were shifted to the left with increased maximum responses. Tracazolate has a unique pharmacological profile on recombinant GABA(A) receptors: its potency (EC(50)) is influenced by the nature of the beta subunit; but more importantly, its intrinsic efficacy, potentiation, or inhibition is determined by the nature of the third subunit (gamma1-3, delta, or epsilon) within the receptor complex.
Enhancement of benzodiazepine and GABA binding by the novel anxiolytic, tracazolate.[Pubmed:6121710]
Eur J Pharmacol. 1982 Mar 12;78(3):315-22.
Tracazolate (ICI 136,753) 4-butylamine-1-ethyl-6-methyl-1H-pyrazolo[3,4]pyridine-5-carboxylic acid ethyl ester is a non-benzodiazepine with anxiolytic-like activity in animal models. In contrast to the benzodiazepines, it enhances [3H]flunitrazepam binding in rat synaptic membrane fragments. The enhancement is potential by chloride ion and is due to an increase in affinity of the receptor. The enhancement of benzodiazepine binding by gamma-aminobutyric acid (GABA) is additive with that of tracazolate; however, the GABA antagonist bicuculline blocks the enhancement by both compounds. Tracazolate enhances [3H]GABA binding to frozen and thawed Triton X-100-treated membrane fragments. The enhancement is due to an increase in the number of sites and potentiated by chloride. Benzodiazepines also enhanced GABA binding but the effect was due to an apparent change in affinity and not potentiated by chloride. The rank order to chlorodiazepoxide, diazepam and flunitrazepam for enhancement of GABA binding and displacement of [3H]flunitrazepam binding were the same. The enhancement of [3H]GABA binding by flunitrazepam and tracazolate were additive. Possible interactions between these various receptors are discussed.
Pharmacological properties of tracazolate: a new non-benzodiazepine anxiolytic agent.[Pubmed:6121711]
Eur J Pharmacol. 1982 Mar 12;78(3):323-33.
Tracazolate (ICI 136,753, 4-butylamino-1-ethyl-6-methyl-1H-pyrazolo[3,4-b]pyridine-5-carboxylic acid ethyl ester) demonstrated dose-related anticonflict activity in rats and mice. The potency of tracazolate appears to be one-quarter to one-half that of chlordiazepoxide. No tolerance to the anticonflict activity of either tracazolate or chlordiazepoxide was evident following 12 consecutive days of treatment. Tracazolate exhibits a much greater separation between sedative and therapeutic doses than does chlordiazepoxide. Furthermore, based on rodent studies, tracazolate should be much less likely than the benzodiazepines to potentiate the actions of barbiturates and ethanol in man. Tracazolate potentiated both the anticonvulsant and anxiolytic effects of chlordiazepoxide in rodents. Unlike benzodiazepines, tracazolate enhances the binding of benzodiazepines to its receptor site. These results suggest that tracazolate is a novel agent with potential clinical utility as an anxiolytic drug.


