3,3'-DiindolylmethaneAnticancer and antineoplastic agent CAS# 1968-05-4 |
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1968-05-4 | SDF | Download SDF |
| PubChem ID | 3071 | Appearance | Powder |
| Formula | C17H14N2 | M.Wt | 246.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 3,3-Diindolylmethane | ||
| Solubility | DMSO : ≥ 100 mg/mL (405.99 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 3-(1H-indol-3-ylmethyl)-1H-indole | ||
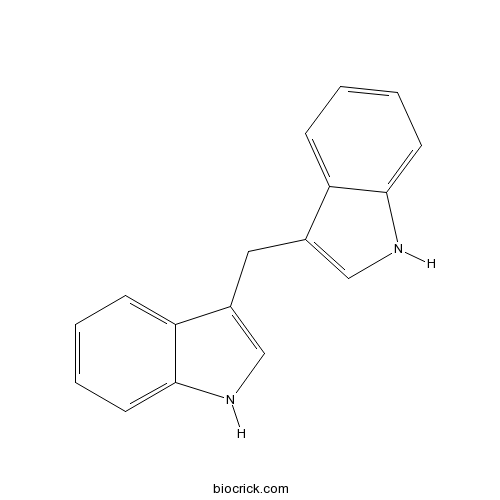
| SMILES | C1=CC=C2C(=C1)C(=CN2)CC3=CNC4=CC=CC=C43 | ||
| Standard InChIKey | VFTRKSBEFQDZKX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14N2/c1-3-7-16-14(5-1)12(10-18-16)9-13-11-19-17-8-4-2-6-15(13)17/h1-8,10-11,18-19H,9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Activator of Chk2 that causes G2/M cell cycle arrest in various cancer cell lines. Antiproliferative and inducer of apoptosis; promotes proteasomal degradation of Cdc25C and Cdk1. Inhibits phosphorylation of EGFR and downstream activation of ERK. |

3,3'-Diindolylmethane Dilution Calculator

3,3'-Diindolylmethane Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0599 mL | 20.2996 mL | 40.5992 mL | 81.1985 mL | 101.4981 mL |
| 5 mM | 0.812 mL | 4.0599 mL | 8.1198 mL | 16.2397 mL | 20.2996 mL |
| 10 mM | 0.406 mL | 2.03 mL | 4.0599 mL | 8.1198 mL | 10.1498 mL |
| 50 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.406 mL | 0.812 mL | 1.015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
3,3'-Diindolylmethane is an anticancer agent [1].
3,3'-Diindolylmethane (DIM) is a dimer of indole-3-carbinol generated in low pH environment. It inhibited cell growth with IC50 values of 17, 24 and 30 μM in MCF-7, T47D and Saos2 cell lines, respectively. DIM induced apoptosis in these cells without affecting the p53 pathway. In human nasopharyngeal carcinoma (NPC) cell line (5-8F NPC), treatment of DIM decreased the ability of cell migration and invasion dose-dependently. In rats, transplanted with NPC cells, DIM administration resulted in suppressing lymph node metastasis. Besides that, DIM was found to induce ERα target gene expression and the concomitant cell proliferation at low concentration (10μM) in the absence of E2 [1, 2 and 3].
References:
1. Ge X, Yannai S, Rennert G, et al. 3, 3′-Diindolylmethane induces apoptosis in human cancer cells. Biochemical and Biophysical Research Communications, 1996, 228(1): 153-158.
2. Wu T, Chen C, Li F, et al. 3, 3' Diindolylmethane inhibits the invasion and metastasis of nasopharyngeal carcinoma cells in vitro and in vivo by regulation of epithelial mesenchymal transition. Experimental and Therapeutic Medicine, 2014, 7(6): 1635-1638.
3. Marques M, Laflamme L, Benassou I, et al. Low levels of 3, 3 [prime]-diindolylmethane activate estrogen receptor alpha and induce proliferation of breast cancer cells in the absence of estradiol. BMC cancer, 2014, 14(1): 524.
- Murrayone
Catalog No.:BCN5331
CAS No.:19668-69-0
- Panaxadiol
Catalog No.:BCN1080
CAS No.:19666-76-3
- Physarorubinic acid A
Catalog No.:BCN1851
CAS No.:196621-49-5
- Giffonin R
Catalog No.:BCN8116
CAS No.:1966183-72-1
- Oseltamivir
Catalog No.:BCC1825
CAS No.:196618-13-0
- BIBX 1382
Catalog No.:BCC1418
CAS No.:196612-93-8
- 4-Acetyl Ramelteon
Catalog No.:BCC1107
CAS No.:1346598-94-4
- 2S-Amino-3R-octadecanol
Catalog No.:BCN1775
CAS No.:196497-48-0
- (RS)-3,5-DHPG
Catalog No.:BCC6613
CAS No.:19641-83-9
- (4S,5R)-3-tert-butoxycarbony-2-(4-anisy)-4-phenyl-5-oxazolidinecarboxylic acid
Catalog No.:BCN8365
CAS No.:196404-55-4
- Siegesmethyethericacid
Catalog No.:BCC9248
CAS No.:196399-16-3
- Prosaptide TX14(A)
Catalog No.:BCC8020
CAS No.:196391-82-9
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- 25R-Inokosterone
Catalog No.:BCN3874
CAS No.:19682-38-3
- (S)-10-Hydroxycamptothecin
Catalog No.:BCN1225
CAS No.:19685-09-7
- 10-Methoxycamptothecin
Catalog No.:BCN2303
CAS No.:19685-10-0
- PQ 401
Catalog No.:BCC1159
CAS No.:196868-63-0
- Athidathion
Catalog No.:BCC5469
CAS No.:19691-80-6
- CD 3254
Catalog No.:BCC7637
CAS No.:196961-43-0
- SB 221284
Catalog No.:BCC7040
CAS No.:196965-14-7
- 7,4-Di-O-methylapigenin 5-O-glucoside
Catalog No.:BCN1508
CAS No.:197018-71-6
- 2-Acetyl-1H-Isoindole-1,3(2H)-Dione
Catalog No.:BCC8511
CAS No.:1971-49-9
- Stigmasterol glucoside
Catalog No.:BCN4865
CAS No.:19716-26-8
- 8-Oxypalmatine
Catalog No.:BCN3137
CAS No.:19716-59-7
3,3'-diindolylmethane mitigates total body irradiation-induced hematopoietic injury in mice.[Pubmed:27609226]
Free Radic Biol Med. 2016 Oct;99:463-471.
We have reported that hematopoietic system injury induced by total body irradiation (TBI) leads to generation of intracellular reactive oxygen species (ROS) and DNA damage, which are ameliorated by antioxidant agents. In the present study, we reported that administration of DIM, a potent antioxidant agent, not only protected mice against TBI-induced lethality, also ameliorated TBI-induced hematopoietic injury. The latter effect was probably attributable to DIM's inhibition of TBI-induced increases in ROS production in hematopoietic stem cells (HSCs) and the phosphorylation of histone H2AX (gamma-H2AX). In particular, DIM led to significant improvements in bone marrow (BM) HSC frequency, hematopoietic progenitor cell (HPC) clonogenic function, and multilineage engraftment after transplantation. A downregulation of NADPH oxidase 4 (NOX4) and an upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) expression were observed following DIM treatment. Notably, the anti-apoptotic potential of DIM was correlated with increased expression of the anti-apoptotic protein Bcl-2 and decreased expression of the pro-apoptotic protein Bax. These findings suggest that DIM attenuates TBI-induced hematopoietic injury through the inhibition of both oxidative stress in HSCs and hematopoietic cell apoptosis. Furthermore, we demonstrated that DIM protected BM hematopoietic cells against ionizing radiation and led to increased clonogenicity in vitro. Therefore, DIM has the potential to be used as an effective radioprotectant to ameliorate TBI-induced hematopoietic injury.
Linking genomic responses of gonads with reproductive impairment in marine medaka (Oryzias melastigma) exposed chronically to the chemopreventive and antifouling agent, 3,3'-diindolylmethane (DIM).[Pubmed:28063342]
Aquat Toxicol. 2017 Feb;183:135-143.
3,3'-Diindolylmethane (DIM) has been promoted as an effective chemopreventive and antifouling additive. However, the concurrent risks or side effects of DIM are not fully understood, especially on tissues responsive to estrogen. Therefore, this study employed marine medaka (Oryzias melastigma) as a test model to evaluate relative safety and explore mechanisms of toxic action of DIM on development and function of gonad after chronic (28days) aqueous exposure to relatively low doses (0mug/L or 8.5mug/L). Integration of comprehensive toxicogenomic analysis at the transcriptome and proteome levels with apical endpoints, such as production of eggs and swimming performance of larvae, elucidated the molecular linkage in gonad from bottom up along the reproductive adverse outcome pathway. A series of sequential changes at the transcript and protein levels were linked to lesser fecundity and viability of larvae exposed to DIM. Anomalous production of vitellogenin (VTG) and eggshell proteins in testis confirmed the estrogenic potency of DIM. In the ovary, although storage of VTG was greater, lesser expressions of cathepsin enzymes blocked cleavage and incorporation of VTG into oocytes as yolk, which acted together with lower eggshell proteins to inhibit maturation of primary oocyte and thus contributed to impairment of fecundity. Overall, this study demonstrated that exposure to DIM impaired reproductive fitness. Diverse molecular initiating changes in gonads were linked to apical endpoints that could be used in assessment of risks posed by DIM on gametogenesis. In combination with chemical stability and potent endocrine disruption, the results of this study can inform decisions about the use of DIM either as chemopreventive or antifouling agent.
Effect of Oral Administration of 3,3'-Diindolylmethane on Dextran Sodium Sulfate-Induced Acute Colitis in Mice.[Pubmed:27700072]
J Agric Food Chem. 2016 Oct 19;64(41):7702-7709.
In patients with inflammatory bowel disease (IBD), inflammation is induced and maintained by lymphangiogenesis and angiogenesis. 3,3'-Diindolylmethane (DIM) is a natural product formed in acidic conditions from indole-3-carbinol in cruciferous vegetables, and it is known for its chemotherapeutic activity. This study evaluated DIM's effects on angiogenesis, lymphangiogenesis, and inflammation in a mouse colitis model. Experimental colitis was induced in mice by administering 3% dextran sulfate sodium (DSS) via drinking water. DIM remarkably attenuated the clinical signs and histological characteristics in mice with DSS-induced colitis. DIM suppressed neutrophil infiltration and pro-inflammatory cytokines. Moreover, it significantly suppressed the expression of vascular endothelial growth factor (VEGF)-A and VEGF receptor (VEGFR)-2, indicating that the mechanism may be related to the repression of pro-angiogenesis activity. DIM also remarkably suppressed the expression of VEGF-C, VEGF-D, VEGFR-3, and angiopoietin-2; thus, the mechanism may also be related to the suppression of lymphangiogenesis. Therefore, DIM is a possible treatment option for inflammation of the intestine and associated angiogenesis and lymphangiogenesis.
Harnessing the Power of Cruciferous Vegetables: Developing a Biomarker for Brassica Vegetable Consumption Using Urinary 3,3'-Diindolylmethane.[Pubmed:27538743]
Cancer Prev Res (Phila). 2016 Oct;9(10):788-793.
Glucobrassicin in Brassica vegetables gives rise to indole-3-carbinol (I3C), a compound with potent anticancer effects in preclinical models. We previously showed that the urinary metabolite 3,3'-diindolylmethane (DIM) could discriminate between volunteers fed high and low doses of Brassica vegetables. However, the quantitative relationship between glucobrassicin exposure and urinary DIM level is unclear. We conducted a clinical trial to examine the hypotheses that a range of glucobrassicin exposure from Brassica vegetables is reflected in urinary DIM and that this effect plateaus. Forty-five subjects consumed vegetables, a mixture of brussels sprouts and/or cabbage, at one of seven discrete dose levels of glucobrassicin ranging from 25 to 500 mumol, once daily for 2 consecutive days. All urine was collected for 24 hours after each vegetable-eating session. Urinary DIM was measured using our published liquid chromatography-electrospray ionization-tandem mass spectrometry-selected reaction monitoring (LC/ESI-MS/MS-SRM) method. Urinary DIM excretion increased predictably with increasing glucobrassicin dose and plateaued between 200 and 300 mumol of glucobrassicin. The association between glucobrassicin dose and urinary DIM was strong and positive (R(2) = 0.68). The majority of DIM was excreted in the first 12 hours after vegetable consumption. We conclude that urinary DIM is a reliable biomarker of glucobrassicin exposure and I3C uptake and that feeding glucobrassicin beyond 200 mumol did not consistently lead to more urinary DIM, suggesting a plateau in potential chemopreventive benefit. Cancer Prev Res; 9(10); 788-93. (c)2016 AACR.
Blocking epidermal growth factor receptor activation by 3,3'-diindolylmethane suppresses ovarian tumor growth in vitro and in vivo.[Pubmed:22205686]
J Pharmacol Exp Ther. 2012 Apr;341(1):24-32.
Genetic alterations, including the overexpression of epidermal growth factor receptor (EGFR) (in approximately 70% of ovarian tumors), play a crucial role in the signal transduction pathways that regulate key cellular functions, such as cell survival and proliferation, and are responsible for compromising traditional chemotherapy. 3,3'-Diindolylmethane (DIM) is an indole compound present in Brassica vegetables. In our previous studies, we demonstrated that BR-DIM, a formulated version of DIM, suppressed the growth of ovarian cancer cells by causing cell cycle arrest and apoptosis. In the present study, we delineated the mechanism by which DIM suppressed the growth of SKOV-3, OVCAR-3, and TOV-21G human ovarian cancer cells. DIM treatment caused significant down-regulation of the constitutive EGFR protein level as well as phosphorylation of EGFR at Tyr1068, Tyr992, Tyr845, and Tyr1173 in various ovarian cancer cells. To determine whether DIM suppressed the activation of EGFR by activating phosphorylation, cells were treated with epidermal growth factor. Epidermal growth factor treatment significantly blocked the DIM-mediated inhibition of EGFR activation and apoptosis in both SKOV-3 and OVCAR-3 cells. In addition, DIM treatment drastically reduced the phosphorylation of mitogen-activated protein kinase kinase (MEK) and extracellular signal-regulated kinase (ERK), which are downstream to EGFR, without affecting their protein levels. DIM treatment also inhibited the kinase activity of ERK, as observed by the down-regulation of phospho-E twenty-six like transcription factor 1 (p-ELK1) in all three ovarian cancer cell lines. DIM significantly suppressed the growth of ovarian tumors in vivo. Tumor growth suppressive effects of DIM in SKOV-3 tumor xenografts were associated with reduced phosphorylation of EGFR, MEK, and ERK. These results indicate that DIM induces apoptosis in ovarian cancer cells by inhibiting the EGFR-ERK pathway in vitro and in vivo.
3,3'-Diindolylmethane induces G1 arrest and apoptosis in human acute T-cell lymphoblastic leukemia cells.[Pubmed:22514694]
PLoS One. 2012;7(4):e34975.
Certain bioactive food components, including indole-3-carbinol (I3C) and 3,3'-diindolylmethane (DIM) from cruciferous vegetables, have been shown to target cellular pathways regulating carcinogenesis. Previously, our laboratory showed that dietary I3C is an effective transplacental chemopreventive agent in a dibenzo[def,p]chrysene (DBC)-dependent model of murine T-cell lymphoblastic lymphoma. The primary objective of the present study was to extend our chemoprevention studies in mice to an analogous human neoplasm in cell culture. Therefore, we tested the hypothesis that I3C or DIM may be chemotherapeutic in human T-cell acute lymphoblastic leukemia (T-ALL) cells. Treatment of the T-ALL cell lines CCRF-CEM, CCRF-HSB2, SUP-T1 and Jurkat with DIM in vitro significantly reduced cell proliferation and viability at concentrations 8- to 25-fold lower than the parent compound I3C. DIM (7.5 microM) arrested CEM and HSB2 cells at the G(1) phase of the cell cycle and 15 microM DIM significantly increased the percentage of apoptotic cells in all T-ALL lines. In CEM cells, DIM reduced protein expression of cyclin dependent kinases 4 and 6 (CDK4, CDK6) and D-type cyclin 3 (CCND3); DIM also significantly altered expression of eight transcripts related to human apoptosis (BCL2L10, CD40LG, HRK, TNF, TNFRSF1A, TNFRSF25, TNFSF8, TRAF4). Similar anticancer effects of DIM were observed in vivo. Dietary exposure to 100 ppm DIM significantly decreased the rate of growth of human CEM xenografts in immunodeficient SCID mice, reduced final tumor size by 44% and increased the apoptotic index compared to control-fed mice. Taken together, our results demonstrate a potential for therapeutic application of DIM in T-ALL.
Activation of checkpoint kinase 2 by 3,3'-diindolylmethane is required for causing G2/M cell cycle arrest in human ovarian cancer cells.[Pubmed:20444961]
Mol Pharmacol. 2010 Aug;78(2):297-309.
We evaluated the effect of 3,3'-diindolylmethane (DIM) in ovarian cancer cells. DIM treatment inhibited the growth of SKOV-3, TOV-21G, and OVCAR-3 ovarian cancer cells in both a dose- and time-dependent manner with effective concentrations ranging from 40 to 100 muM. Growth-inhibitory effects of DIM were mediated by cell cycle arrest in G(2)/M phase in all the three cell lines. G(2)/M arrest was associated with DNA damage as indicated by phosphorylation of H(2)A.X at Ser139 and activation of checkpoint kinase 2 (Chk2) in all the three cell lines. Other G(2)/M regulatory molecules such as Cdc25C, Cdk1, cyclin B1 were down-regulated by DIM. Cycloheximide or Chk2 inhibitor pretreatment abrogated not only activation of Chk2 but also G(2)/M arrest and apoptosis mediated by DIM. To further establish the involvement of Chk2 in DIM-mediated G(2)/M arrest, cells were transfected with dominant-negative Chk2 (DN-Chk2). Blocking Chk2 activation by DN-Chk2 completely protected cells from DIM-mediated G(2)/M arrest. These results were further confirmed in Chk2 knockout DT40 lymphoma cells, in which DIM failed to cause cell cycle arrest. These results clearly indicate the requirement of Chk2 activation to cause G(2)/M arrest by DIM in ovarian cancer cells. Moreover, blocking Chk2 activation also abrogates the apoptosis-inducing effects of DIM. Furthermore, our results show that DIM treatment cause ROS generation. Blocking ROS generation by N-acetyl cysteine protects the cells from DIM-mediated G(2)/M arrest and apoptosis. Our results establish Chk2 as a potent molecular target of DIM in ovarian cancer cells and provide the rationale for further clinical investigation of DIM.


