Oseltamivirinhibitor of influenza neuraminidase CAS# 196618-13-0 |
- Zanamivir
Catalog No.:BCC4946
CAS No.:139110-80-8
- Oseltamivir acid
Catalog No.:BCC1826
CAS No.:187227-45-8
- Oseltamivir phosphate
Catalog No.:BCC4690
CAS No.:204255-11-8
- Peramivir
Catalog No.:BCC1846
CAS No.:330600-85-6
- Nucleozin
Catalog No.:BCC1811
CAS No.:341001-38-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 196618-13-0 | SDF | Download SDF |
| PubChem ID | 65028 | Appearance | Powder |
| Formula | C16H28N2O4 | M.Wt | 312.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >31.2mg/ml in DMSO | ||
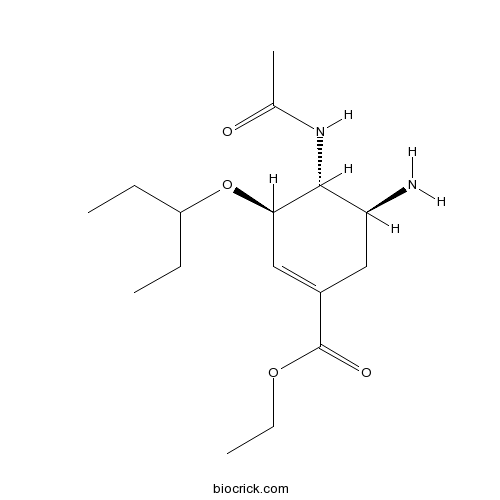
| Chemical Name | ethyl (3R,4R,5S)-4-acetamido-5-amino-3-pentan-3-yloxycyclohexene-1-carboxylate | ||
| SMILES | CCC(CC)OC1C=C(CC(C1NC(=O)C)N)C(=O)OCC | ||
| Standard InChIKey | VSZGPKBBMSAYNT-RRFJBIMHSA-N | ||
| Standard InChI | InChI=1S/C16H28N2O4/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19/h9,12-15H,5-8,17H2,1-4H3,(H,18,19)/t13-,14+,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Oseltamivir Dilution Calculator

Oseltamivir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.201 mL | 16.0051 mL | 32.0102 mL | 64.0205 mL | 80.0256 mL |
| 5 mM | 0.6402 mL | 3.201 mL | 6.402 mL | 12.8041 mL | 16.0051 mL |
| 10 mM | 0.3201 mL | 1.6005 mL | 3.201 mL | 6.402 mL | 8.0026 mL |
| 50 mM | 0.064 mL | 0.3201 mL | 0.6402 mL | 1.2804 mL | 1.6005 mL |
| 100 mM | 0.032 mL | 0.1601 mL | 0.3201 mL | 0.6402 mL | 0.8003 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Oseltamivir is an inhibitor of influenza neuraminidase [1].
Oseltamivir is a prodrug that is converted by intestinal and/or hepatic esterases to the neuraminidase inhibitor molecule, oseltamivir carboxylate. Neuraminidase cleaves the terminal a-Neu5Ac residues from the newly synthesized virion progeny and let it elute from the infected cell and seek new host cells to infect. Oseltamivir efficiently block sialidase activity and significantly inhibit the releasing mechanism [1].
In the treatment of adults, oseltamivir reduces the time to first alleviation of symptoms and investigator mediated unverified pneumonia. In prophylaxis trials, oseltamivir reduced symptomatic influenza in participants by 55%. Oseltamivir also has some harm. Adults treated with oseltamivir are associated with an increased risk of nausea. And in prophylaxis trials there is an increased risk of headaches on-treatment [2].
As a neuraminidase inhibitor, the substitution of the amino acid histidine to tyrosine at position 275 (H275Y) in the neuraminidase gene of H1N1 can cause the resistance of oseltamivir [3].
References:
[1]. Enguang Feng, Deju Ye, Jian Li, Dengyou Zhang, Jinfang Wang, Fei Zhao, Rolf Hilgenfeld, Mingyue Zheng, Hualiang Jiang and Hong Liu. Recent Advances in Neuraminidase Inhibitor Development as Anti-influenza Drugs. Chem Med Chem, 2012, 7: 1527-1536.
[2]. Tom Jefferson reviewer, Mark Jones, Peter Doshi, Elizabeth A Spencer, Igho Onakpoya, Carl J Heneghan. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comment. BMJ, 2014, 348: g2545.
[3]. Rashmi Dixit, Gulam Khandaker, Scott Ilgoutz, Harunor Rashid and Robert Booy. Emergence of Oseltamivir Resistance: Control and Management of Influenza before, during and after the Pandemic. Infectious Disorders-Drug Targets. 2013, 13 (1): 34-45.
- BIBX 1382
Catalog No.:BCC1418
CAS No.:196612-93-8
- 4-Acetyl Ramelteon
Catalog No.:BCC1107
CAS No.:1346598-94-4
- 2S-Amino-3R-octadecanol
Catalog No.:BCN1775
CAS No.:196497-48-0
- (RS)-3,5-DHPG
Catalog No.:BCC6613
CAS No.:19641-83-9
- (4S,5R)-3-tert-butoxycarbony-2-(4-anisy)-4-phenyl-5-oxazolidinecarboxylic acid
Catalog No.:BCN8365
CAS No.:196404-55-4
- Siegesmethyethericacid
Catalog No.:BCC9248
CAS No.:196399-16-3
- Prosaptide TX14(A)
Catalog No.:BCC8020
CAS No.:196391-82-9
- Axillarine
Catalog No.:BCN2059
CAS No.:19637-66-2
- Bay 11-7085
Catalog No.:BCC5105
CAS No.:196309-76-9
- 4-Methoxycinnamaldehyde
Catalog No.:BCN2700
CAS No.:1963-36-6
- Indole-3-acrylic acid methyl ester
Catalog No.:BCN1190
CAS No.:19626-92-7
- Angelol A
Catalog No.:BCN8036
CAS No.:19625-17-3
- Giffonin R
Catalog No.:BCN8116
CAS No.:1966183-72-1
- Physarorubinic acid A
Catalog No.:BCN1851
CAS No.:196621-49-5
- Panaxadiol
Catalog No.:BCN1080
CAS No.:19666-76-3
- Murrayone
Catalog No.:BCN5331
CAS No.:19668-69-0
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- 25R-Inokosterone
Catalog No.:BCN3874
CAS No.:19682-38-3
- (S)-10-Hydroxycamptothecin
Catalog No.:BCN1225
CAS No.:19685-09-7
- 10-Methoxycamptothecin
Catalog No.:BCN2303
CAS No.:19685-10-0
- PQ 401
Catalog No.:BCC1159
CAS No.:196868-63-0
- Athidathion
Catalog No.:BCC5469
CAS No.:19691-80-6
- CD 3254
Catalog No.:BCC7637
CAS No.:196961-43-0
Aerosol administration increases the efficacy of oseltamivir for the treatment of mice infected with influenza viruses.[Pubmed:28286235]
Antiviral Res. 2017 Jun;142:12-15.
Oseltamivir is an influenza neuraminidase inhibitor that along with supportive therapy has shown to help critically ill patients infected with H7N9 and H1N1pdm influenza virus strains to recover from disease. The standard of care recommends the administration of Oseltamivir via oral route which represents difficulties in patients with gastrointestinal complications. Here we tested the use of aerosol administration of Oseltamivir to treat mice infected with influenza A/H7N9 virus or influenza A/H1N1pdm virus and directly compared this approach to the standard of care, oral administration. Using nose only delivery of aerosolized Oseltamivir we observed a significant increase in efficacy of the treatment compared to oral administration characterized by reduced body weight loss, increased survival rate and dose sparing. The preclinical data presented here supports the possibility of using this approach in clinical settings.
Prospective surveillance of antiviral resistance in hospitalized infants less than 12 months of age with A(H3N2) influenza infection and treated with oseltamivir.[Pubmed:28205506]
Antivir Ther. 2017;22(6):515-522.
BACKGROUND: Infants exhibit elevated influenza virus loads and prolonged viral shedding, which may increase the risk for resistance development, especially in cases of suboptimal exposure to antiviral therapy. METHODS: We performed a prospective surveillance of hospitalized infants undergoing Oseltamivir therapy during the 2008-2009 and 2011-2012 influenza seasons at two paediatric hospitals in Germany. A total of 37 infants less than 1 year of age with laboratory confirmed influenza A(H3N2) infection received Oseltamivir as per physician's order for 5 days (2008-2009 season: 2 mg/kg twice daily; 2011-2012 season: 2.0 mg/kg; 2.5 mg/kg and 3.0 mg/kg twice daily for infants <1 month; 2-3 months and 4-12 months, respectively). Virus load, the susceptibility to neuraminidase inhibitors (NAIs), and the presence of molecular markers of resistance to NAIs was assessed for influenza viruses recovered from respiratory samples collected at baseline and during follow-up visits. RESULTS: Overall, 73% of the infants continued to shed viral RNA detectable by reverse transcription (RT)-PCR after dose number 10 of Oseltamivir; 12 infants shed viruses, 2 of them (both 9 months of age) shed resistant viruses. Resistance was characterized by >/=1,000-fold increase of 50% inhibitory concentration (IC50) for Oseltamivir, up to 50-fold for zanamivir and elevated Km values when compared to susceptible A(H3N2) strains. Sanger sequencing revealed the selection of the NA-R292K substitution in both instances (after dose number 10 on day 6). CONCLUSIONS: Our data suggest that it may be relevant to monitor antiviral resistance systematically in all infants, considering that the European Medicines Agency has recently extended the licensure for Oseltamivir to include full-term infants.
Selectivity Improvement for Spectrofluorimetric Determination of Oseltamivir Phosphate in Human Plasma and in the Presence of Its Degradation Product.[Pubmed:28293815]
J Fluoresc. 2017 Jul;27(4):1323-1330.
A simple and sensitive spectrofluorimetric method has been developed and validated for determination of Oseltamivir phosphate (OSP). The proposed method is based on condensation reaction of the primary amino group of OSP with ninhydrin and phenylacetaldehyde in buffered medium (pH 6.5). The formed yellow fluorescent product exhibits excitation and emission maxima at 390 and 460 nm, respectively. The selectivity improvement of our proposed method is based on the water insolubility of the Oseltamivir carboxylic acid (OSC) the active metabolite of OSP, which contains the same primary amino group as OSP but cannot, condensed with ninhydrin and phenylacetaldehyde reagents. The different experimental parameters affecting the formation and stability of the reaction product were carefully studied and optimized. The fluorescence intensity concentration plot is rectilinear in the range of 2-15 mug ml(-1) with detection and quantitation limits of 0.32 and 0.98 mug ml(-1), respectively. The proposed method was successfully applied for determination of OSP in commercial capsules, suspension and spiked human plasma with good percentage recovery. In addition, the developed procedure was extended to study the stability of OSP under different stress conditions; including acid and alkali hydrolysis, oxidation, photolysis, and thermal degradation. Furthermore, the kinetic of alkaline and acidic degradation of the cited drug were investigated. The apparent first order degradation rate constants were 0.258 and 0.318 K h(-1) with half times of 2.68 and 2.17 h, for acidic and alkaline degradation, respectively.
Effects of dexamethasone coadministered with oseltamivir on the pharmacokinetics of oseltamivir in healthy volunteers.[Pubmed:28331290]
Drug Des Devel Ther. 2017 Mar 9;11:705-711.
PURPOSE: Oseltamivir is widely used in the treatment and prophylaxis of influenza A and B viral infections. It is ingested as an oral prodrug that is rapidly metabolized by carboxylesterase 1 (CES1) to its active form, Oseltamivir carboxylate. Dexamethasone is also used in the treatment of acute respiratory distress syndrome, a severe complication of influenza; however, its influence on the pharmacokinetics (PK) of Oseltamivir is controversial. The aim of this study was to investigate the effects of coadministering Oseltamivir and dexamethasone on the PK of Oseltamivir in healthy volunteers. METHODS: An open-label, two-period, one-sequence, multiple-dose study was conducted in 19 healthy male volunteers. Oseltamivir (75 mg) was orally administered on Day 1 and Day 8, and dexamethasone (1.5 mg) was administered once daily from Day 3 to Day 8. Serial blood and urine samples were collected for PK analysis of Oseltamivir and Oseltamivir carboxylate on Day 1 and Day 8. Oseltamivir and Oseltamivir carboxylate concentrations in plasma and urine were determined using liquid chromatography-tandem mass spectrometry. RESULTS: Area under the plasma concentration-time curve (AUC) of Oseltamivir and Oseltamivir carboxylate decreased after dexamethasone treatment for 6 days. The geometric mean ratio (90% confidence interval) of the metabolic ratio (Oseltamivir carboxylate AUC0-48h/Oseltamivir AUC0-48h) was 0.92 (0.87-0.97). The amount of unchanged Oseltamivir excreted in urine increased by 14% after dexamethasone treatments. CONCLUSION: Coadministration of dexamethasone with Oseltamivir slightly decreased systemic exposure to Oseltamivir and Oseltamivir carboxylate in healthy volunteers. This result suggests that CES1 is inhibited by dexamethasone in humans. However, coadministration of Oseltamivir and dexamethasone did not appear to have a clinically relevant effect on the PK of Oseltamivir; based on these results, dexamethasone can be coadministered with Oseltamivir.


