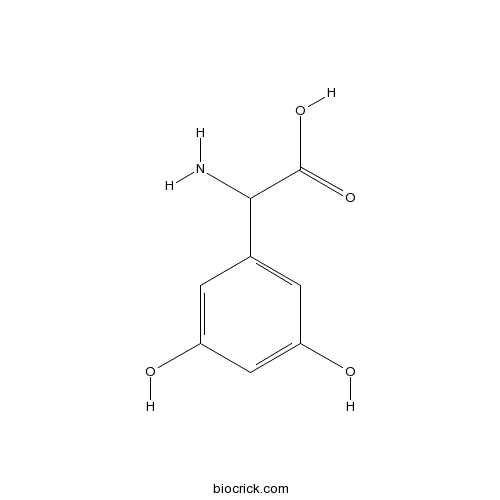
(RS)-3,5-DHPGSelective group I mGlu agonist CAS# 19641-83-9 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19641-83-9 | SDF | Download SDF |
| PubChem ID | 108001 | Appearance | Powder |
| Formula | C8H9NO4 | M.Wt | 183.16 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water | ||
| Chemical Name | 2-amino-2-(3,5-dihydroxyphenyl)acetic acid | ||
| SMILES | C1=C(C=C(C=C1O)O)C(C(=O)O)N | ||
| Standard InChIKey | HOOWCUZPEFNHDT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H9NO4/c9-7(8(12)13)4-1-5(10)3-6(11)2-4/h1-3,7,10-11H,9H2,(H,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective group I metabotropic glutamate receptor agonist which activates both mGlu1 and mGlu5. Also reported to be an antagonist at metabotropic glutamate receptors linked to phospholipase D. (S)-3,5-DHPG also available. |

(RS)-3,5-DHPG Dilution Calculator

(RS)-3,5-DHPG Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4597 mL | 27.2985 mL | 54.5971 mL | 109.1941 mL | 136.4927 mL |
| 5 mM | 1.0919 mL | 5.4597 mL | 10.9194 mL | 21.8388 mL | 27.2985 mL |
| 10 mM | 0.546 mL | 2.7299 mL | 5.4597 mL | 10.9194 mL | 13.6493 mL |
| 50 mM | 0.1092 mL | 0.546 mL | 1.0919 mL | 2.1839 mL | 2.7299 mL |
| 100 mM | 0.0546 mL | 0.273 mL | 0.546 mL | 1.0919 mL | 1.3649 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (4S,5R)-3-tert-butoxycarbony-2-(4-anisy)-4-phenyl-5-oxazolidinecarboxylic acid
Catalog No.:BCN8365
CAS No.:196404-55-4
- Siegesmethyethericacid
Catalog No.:BCC9248
CAS No.:196399-16-3
- Prosaptide TX14(A)
Catalog No.:BCC8020
CAS No.:196391-82-9
- Axillarine
Catalog No.:BCN2059
CAS No.:19637-66-2
- Bay 11-7085
Catalog No.:BCC5105
CAS No.:196309-76-9
- 4-Methoxycinnamaldehyde
Catalog No.:BCN2700
CAS No.:1963-36-6
- Indole-3-acrylic acid methyl ester
Catalog No.:BCN1190
CAS No.:19626-92-7
- Angelol A
Catalog No.:BCN8036
CAS No.:19625-17-3
- 17 alpha-propionate
Catalog No.:BCC1296
CAS No.:19608-29-8
- 25S-Inokosterone
Catalog No.:BCN3873
CAS No.:19595-18-7
- (R)-Nepicastat HCl
Catalog No.:BCC4315
CAS No.:195881-94-8
- HTMT dimaleate
Catalog No.:BCC6736
CAS No.:195867-54-0
- 2S-Amino-3R-octadecanol
Catalog No.:BCN1775
CAS No.:196497-48-0
- 4-Acetyl Ramelteon
Catalog No.:BCC1107
CAS No.:1346598-94-4
- BIBX 1382
Catalog No.:BCC1418
CAS No.:196612-93-8
- Oseltamivir
Catalog No.:BCC1825
CAS No.:196618-13-0
- Giffonin R
Catalog No.:BCN8116
CAS No.:1966183-72-1
- Physarorubinic acid A
Catalog No.:BCN1851
CAS No.:196621-49-5
- Panaxadiol
Catalog No.:BCN1080
CAS No.:19666-76-3
- Murrayone
Catalog No.:BCN5331
CAS No.:19668-69-0
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- 25R-Inokosterone
Catalog No.:BCN3874
CAS No.:19682-38-3
- (S)-10-Hydroxycamptothecin
Catalog No.:BCN1225
CAS No.:19685-09-7
Regulation of phosphoinositide turnover in neonatal rat cerebral cortex by group I- and II- selective metabotropic glutamate receptor agonists.[Pubmed:9504400]
Br J Pharmacol. 1998 Feb;123(3):581-9.
1. The interactive effects of different metabotropic glutamate (mGlu) receptor subtypes to regulate phosphoinositide turnover have been studied in neonatal rat cerebral cortex and hippocampus by use of agonists and antagonists selective between group I and II mGlu receptors. 2, The group II-selective agonist 2R,4R-4-aminopyrrolidine-2,4-dicarboxylate (2R,4R-APDC; 100 microM) had no effect on basal total inositol phosphate ([3H]-InsPx) accumulation (in the presence of Li+) in myo-[3H]-inositol pre-labelled slices, but enhanced the maximal [3H]-InsPx response to the group I-selective agonist (S)-3,5-dihydroxyphenylglycine (DHPG) by about 100% in both hippocampus and cerebral cortex. In cerebral cortex the enhancing effect of 2R,4R-APDC occurred with respect to the maximal responsiveness and had no effect on EC50 values for DHPG (-log EC50 (M): control, 5.56+/-0.05; +2R,4R-APDC, 5.51+/-0.08). 2R,4R-APDC also caused a significant enhancement of the DHPG-stimulated inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) mass response over an initial 0-300 s time-course. 3. The enhancing effects of 2R,4R-APDC on DHPG-stimulated [3H]-InsPx accumulation were observed in both the presence and nominal absence of extracellular Ca2+, and irrespective of whether 2R,4R-APDC was added before, simultaneous with, or subsequent to DHPG. Furthermore, increasing the tissue cyclic AMP concentration up to 100 fold had no effect on DHPG-stimulated Ins(l,4,5)P3 accumulation in the absence or presence of 2R,4R-APDC. 4. 2R,4R-APDC and (2S, 1'R, 2'R, 3'R)-2-(2,3-dicarboxylcyclopropyl)glycine (DCG-IV), the latter agent in the presence of MK-801 to prevent activation of NMDA-receptors, each inhibited forskolin-stimulated cyclic AMP accumulation by about 50%, with respective EC50 values of 1.3 and 0.04 microM (-log EC 50 (M): 2R,4R-APDC, 5.87+/-0.09; DCG-IV, 7.38+/-0.05). In the presence of DHPG (30 microM), 2R,4R-APDC and DCG-IV also concentration-dependently increased [3H]-InsPx accumulation with respective EC50 values of 4.7 and 0.28 microM (-log EC50 (M): 2R,4R-APDC, 5.33+/-0.04; DCG-IV, 6.55+/-0.09) which were 3-7 fold rightward-shifted relative to the adenylyl cyclase inhibitory responses. 5. The group II-selective mGlu receptor antagonist LY307452 (30 microM) caused parallel rightward shifts in the concentration-effect curves for inhibition of forskolin-stimulated adenylyl cyclase, and enhancement of DHPG-stimulated [3H]-InsPx accumulation, by 2R,4R-APDC yielding similar equilibrium dissociation constants (KdS, 3.7+/-1.1 and 4.1+/-0.4 microM respectively) for each response. 6. The ability of 2R,4R-APDC to enhance receptor-mediated [3H]-InsPx accumulation appeared to be agonist-specific; thus although DHPG (100 microM) and the muscarinic cholinoceptor agonist carbachol (10 microM) stimulated similar [3H]-InsPx accumulations, only the response to the former agonist was enhanced by co-activation of group II mGlu receptors. 7. These data demonstrate that second messenger-generating phosphoinositide responses stimulated by group I mGlu receptors are positively modulated by co-activation of group II mGlu receptors in cerebral cortex and hippocampus. The data presented here are discussed with respect to the possible mechanisms which might mediate the modulatory activity, and the physiological and pathophysiological significance of such crosstalk between mGlu receptors.
The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus.[Pubmed:9517422]
Neuropharmacology. 1997 Nov-Dec;36(11-12):1517-32.
The group I specific metabotropic glutamate (mGlu) receptor agonist (RS)-3,5-dihydroxyphenylglycine (DHPG) (100 microM, 10 min) induced long-term depression (LTD) of synaptic transmission in the CA1 region of adult rat hippocampal slices, measured using a grease-gap recording technique. In "normal" (1 mM Mg2+-containing) medium, LTD (measured 30 min after washout of DHPG) was small (13+/-3%), but LTD was enhanced if DHPG was applied when the tissue was made hyperexcitable, either by omitting Mg2+ from the perfusate (35+/-3%) or by adding the GABA(A) receptor antagonist picrotoxin (29+/-2%). The N-methyl-D-aspartate (NMDA) receptor antagonist AP5 (100 microM) substantially reduced the generation of DHPG-induced LTD in Mg2+-free medium, but had little effect on LTD induced in the presence of picrotoxin. In Mg2+-free medium, the threshold concentration of DHPG required to induce LTD was between 1 and 3 microM. Neither agonists specific for group II (100 nM DCG-IV or 1 microM LY354740) or group III (10 microM L-AP4) mGlu receptors or a combined group I and II agonist (30-100 microM (1S,3R)-ACPD) induced LTD. However, an agonist (1 mM CHPG) which activates mGlu5 but not mGlu1 receptors did induce LTD. Surprisingly, DHPG-induced LTD was reversed by mGlu receptor antagonists, applied hours after washout of DHPG. DHPG-induced LTD did not occlude with LTD induced by synaptic activation (1200 stimuli delivered at 2 Hz), in Mg2+-free medium. These data show that activation of group I mGlu receptors (probably mGlu5) can induce LTD and that this mGlu receptor-mediated LTD may, or may not, require activation of NMDA receptors, depending on the experimental conditions.
Pharmacological characterization of metabotropic glutamate receptors coupled to phospholipase D in the rat hippocampus.[Pubmed:8799579]
Br J Pharmacol. 1996 Jun;118(4):1035-43.
1. Phospholipase D (PLD) is the key enzyme in a signal transduction pathway leading to the formation of the second messengers phosphatidic acid and diacylglycerol. In order to define the pharmacological profile of PLD-coupled metabotropic glutamate receptors (mGluRs), PLD activity was measured in slices of adult rat brain in the presence of mGluR agonists or antagonists. Activation of the phospholipase C (PLC) pathway by the same agents was also examined. 2. The mGluR-selective agonist (1S,3R)-l-aminocyclopentane-1,3-dicarboxylic acid [(1S,3R)-ACPD] induced a concentration-dependent (10-300 microM) activation of PLD in the hippocampus, neocortex, and striatum, but not in the cerebellum. The effect was particularly evident in hippocampal slices, which were thus used for all subsequent experiments. 3. The rank order of potencies for agonists stimulating the PLD response was: quisqualate > ibotenate > (2S,3S,4S)-alpha-(carboxycyclopropyl)-glycine > (1S,3R)-ACPD > L-cysteine sulphinic acid > L-aspartate > L-glutamate. L-(+)-2-Amino-4-phosphonobutyric acid and the ionotropic glutamate receptor agonists N-methyl-D-aspartate, alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, and kainate failed to activate PLD. (RS)-3,5-dihydroxyphenylglycine (100300 microM), an agonist of mGluRs of the first group, stimulated PLC but inhibited the PLD response elicited by 100 microM (1S,3R)-ACPD. 4. (+)-alpha-Methyl-4-carboxyphenylglycine (0.1-1 mM), a competitive antagonist of mGluRs of the first and second group, elicited a significant PLD response. L-(+)-2-Amino-3-phosphonopropionic acid (1 mM), an antagonist of mGluRs of the first group, inhibited the 100 microM (1S,3R)-ACPD-induced PLC response but produced a robust stimulation of PLD. 5. 12-O-Tetradecanoylphorbol 13-acetic acid and phorbol 12,13-dibutyrate (PDBu), activators of protein kinase C, at 1 microM had a stimulatory effect on mGluRs linked to PLD but depressed (1S,3R)-ACPD-induced phosphoinositide hydrolysis. The protein kinase C inhibitor, staurosporine (1 and 10 microM) reduced PLD activation induced by 1 microM PDBu but not by 100 microM (1S,3R)-ACPD. 6. Our results suggest that PLD-linked mGluRs in rat hippocampus may be distinct from any known mGluR subtype coupled to PLC or adenylyl cyclase. Moreover, they indicate that independent mGluRs coupled to the PLC and PLD pathways exist and that mGluR agonists can stimulate PLD through a PKC-independent mechanism.
3,5-Dihydroxyphenyl-glycine: a potent agonist of metabotropic glutamate receptors.[Pubmed:1362358]
Neuroreport. 1992 Nov;3(11):1013-6.
An amino acid, 3,5-dihydroxyphenylglycine (DHPG) induced current responses in Xenopus oocytes expressing a metabotropic glutamate receptor clone mGluR1. Apparent EC50 of DHPG for mGluR1 was slightly lower than that of (+-)-1-aminocyclopentane-trans-1,3-dicarboxylic acid (ACPD). DHPG responses were partially inhibited by 2-amino-3-phosphonopropionic acid (AP-3). DHPG had no effect on ionotropic glutamate receptors whose expression was induced in the oocytes following injection of poly(A)+ mRNA of rat brains. In hippocampal slices, DHPG produced slow excitation of pyramidal cells, resulting from a depression of Ca(2+)-dependent K+ current and a voltage-dependent K+ current. These results indicate that DHPG is a specific and potent agonist of mGluRs.


