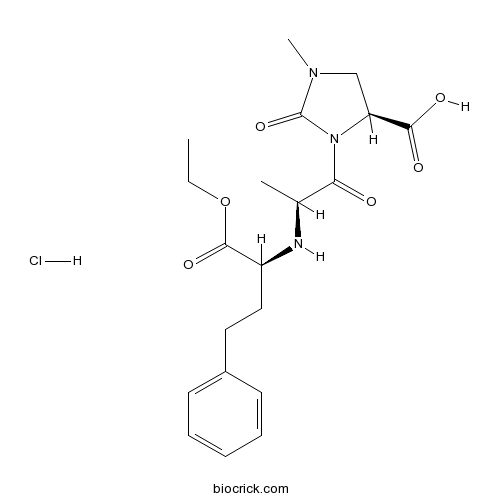
Imidapril HClCAS# 89396-94-1 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Rosuvastatin Calcium
Catalog No.:BCC3853
CAS No.:147098-20-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 89396-94-1 | SDF | Download SDF |
| PubChem ID | 5485193 | Appearance | Powder |
| Formula | C20H28ClN3O6 | M.Wt | 441.91 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TA-6366 | ||
| Solubility | DMSO : 50 mg/mL (113.15 mM; Need ultrasonic) H2O : 50 mg/mL (113.15 mM; Need ultrasonic) | ||
| Chemical Name | (4S)-3-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-1-methyl-2-oxoimidazolidine-4-carboxylic acid;hydrochloride | ||
| SMILES | CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2C(CN(C2=O)C)C(=O)O.Cl | ||
| Standard InChIKey | LSLQGMMMRMDXHN-GEUPQXMHSA-N | ||
| Standard InChI | InChI=1S/C20H27N3O6.ClH/c1-4-29-19(27)15(11-10-14-8-6-5-7-9-14)21-13(2)17(24)23-16(18(25)26)12-22(3)20(23)28;/h5-9,13,15-16,21H,4,10-12H2,1-3H3,(H,25,26);1H/t13-,15-,16-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Imidapril Hydrochloride is the hydrochloride salt of Imidapril, an angiotensin-converting enzyme (ACE) inhibitor with antihypertensive activity. Target: ACE As a prodrug, Imidapril is converted by hydrolysis in the liver into its active form imidaprilat. Imidaprilat competitively binds to and inhibits ACE, thereby blocking the conversion of angiotensin I to angiotensin II. This prevents the potent vasoconstrictive actions of angiotensin II and results in vasodilation. Imidaprilat also decreases angiotensin II-induced aldosterone secretion by the adrenal cortex, which leads to an increase in sodium excretion and subsequently increases water outflow. | |||||

Imidapril HCl Dilution Calculator

Imidapril HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2629 mL | 11.3145 mL | 22.629 mL | 45.2581 mL | 56.5726 mL |
| 5 mM | 0.4526 mL | 2.2629 mL | 4.5258 mL | 9.0516 mL | 11.3145 mL |
| 10 mM | 0.2263 mL | 1.1315 mL | 2.2629 mL | 4.5258 mL | 5.6573 mL |
| 50 mM | 0.0453 mL | 0.2263 mL | 0.4526 mL | 0.9052 mL | 1.1315 mL |
| 100 mM | 0.0226 mL | 0.1131 mL | 0.2263 mL | 0.4526 mL | 0.5657 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Imidapril (INN) is an ACE inhibitor used as an antihypertensive drug and for the treatment of chronic heart failure.
- PF 945863
Catalog No.:BCC6172
CAS No.:893556-85-9
- Riligustilide
Catalog No.:BCC9136
CAS No.:89354-45-0
- Chiisanoside
Catalog No.:BCN2712
CAS No.:89354-01-8
- ICI 174,864
Catalog No.:BCC5675
CAS No.:89352-67-0
- 3,4-O,O-Methylene-(+)-catechin
Catalog No.:BCN7962
CAS No.:89329-14-6
- 2,4-Dihydroxy-3-nitropyridine
Catalog No.:BCC8499
CAS No.:89282-12-2
- MF63
Catalog No.:BCC1744
CAS No.:892549-43-8
- 2-(Chloromethyl)-4-(4-nitrophenyl)-1,3-thiazole
Catalog No.:BCC8372
CAS No.:89250-26-0
- AZD 3988
Catalog No.:BCC5621
CAS No.:892489-52-0
- Manidipine 2HCl
Catalog No.:BCC4405
CAS No.:89226-75-5
- Manidipine
Catalog No.:BCC4404
CAS No.:89226-50-6
- LY2334737
Catalog No.:BCC4060
CAS No.:892128-60-8
- VU 0240551
Catalog No.:BCC5424
CAS No.:893990-34-6
- LDN 212320
Catalog No.:BCC6361
CAS No.:894002-50-7
- Raddeanin A
Catalog No.:BCN1084
CAS No.:89412-79-3
- STF-118804
Catalog No.:BCC4850
CAS No.:894187-61-2
- [D-Trp7,9,10]-Substance P
Catalog No.:BCC7202
CAS No.:89430-38-6
- DMOG
Catalog No.:BCC2433
CAS No.:89464-63-1
- BIBF 1202
Catalog No.:BCC5298
CAS No.:894783-71-2
- ST 2825
Catalog No.:BCC1967
CAS No.:894787-30-5
- TCS JNK 6o
Catalog No.:BCC7607
CAS No.:894804-07-0
- Picrasinol B
Catalog No.:BCN4440
CAS No.:89498-91-9
- Methylneoquassin
Catalog No.:BCN3121
CAS No.:89498-93-1
- SC 144
Catalog No.:BCC1171
CAS No.:895158-95-9
[A Case of Life-Threatening Angioedema Occurred During Prolonged Angiotensin-Converting Enzyme Inhibitor Treatment].[Pubmed:26972946]
J UOEH. 2016 Mar 1;38(1):61-4.
Although angiotensin-converting enzyme (ACE) inhibitors are widely used as the first choice drug for treating hypertension, we have only a superficial understanding of their relationship to angioedema. We report a case of life-threatening angioedema. The case was a 60-year-old man who had been taking an ACE inhibitor for hypertension for 11 years. He visited his home doctor for dyspnea, and tongue and neck swelling. He was transported to our hospital because of the possibility of airway obstruction. On admission, his tongue and neck swelling became more severe. We performed an intubation using an endoscope and started airway management. We also stopped his ACE inhibitor. The severe tongue and neck swelling improved gradually and he was extubated on day 3. On the fifth day he was discharged. We diagnosed angioedema caused by an ACE inhibitor. Although the risk of airway obstruction with ACE inhibitors is acknowledged, we have only a superficial understanding of how prolonged ACE inhibitor treatment induces angioedema. So we should consider angioedema in cases of taking ACE inhibitors, especially in cases of prolonged treatment.
A Comparative Effectiveness Study of Renal Parameters Between Imidapril and Amlodipine in Patients with Hypertension: A Retrospective Cohort Study.[Pubmed:28044266]
Cardiol Ther. 2017 Jun;6(1):69-80.
INTRODUCTION: Imidapril is an angiotensin converting enzyme inhibitor (ACEI) that is frequently used as an antihypertensive drug in Japan. Although ACEIs are known to have adverse effects of decreasing glomerular filtration rate (GFR) and causing hyperkalemia, there are very few clinical data on the long-term effect of imidapril on glomerular function. We conducted a retrospective cohort study using a clinical database to evaluate and compare the long-term effects of imidapril and amlodipine on renal parameters in Japanese hypertensive patients in routine clinical practice. METHODS: We identified cohorts of new users of imidapril (n = 57) and a propensity score-matched group with an equal number of new users of amlodipine (n = 57). We used a multivariable regression model to evaluate and compare the effects of the drugs on laboratory parameters including serum levels of creatinine, potassium, sodium, blood urea nitrogen, and estimated GFR (eGFR) between imidapril users and amlodipine users up to 12 months after the initiation of study drug administration. The mean exposure of imidapril and amlodipine was 226.2 and 235.2 days, respectively. RESULTS: We found a significant increase of serum creatinine and potassium levels and a decrease of eGFR in imidapril users from the baseline period to the exposure period. The reduction of eGFR and the increase of serum creatinine and potassium levels in imidapril users were significantly greater than those in amlodipine users. CONCLUSIONS: Our study showed that imidapril decreased eGFR and increases the serum levels of creatinine and potassium compared with amlodipine, at least during 1 year of administration.
Development and Validation of a Stability-Indicating HPLC Method for Imidapril and Its Degradation Products Using a Design of Experiment (DoE) Approach.[Pubmed:28803600]
J AOAC Int. 2017 Nov 1;100(6):1727-1738.
The present work focused on the application of design of experiment (DoE) principles to the development and optimization of a stability-indicating method (SIM) for the drug imidapril hydrochloride and its degradation products (DPs). The resolution of peaks for the DPs and their drug in a SIM can be influenced by many factors. The factors studied here were pH, gradient time, organic modifier, flow rate, molar concentration of the buffer, and wavelength, with the aid of a Plackett-Burman design. Results from the Plackett-Burman study conspicuously showed influence of two factors, pH and gradient time, on the analyzed response, particularly, the resolution of the closely eluting DPs (DP-5 and DP-6) and the retention time of the last peak. Optimization of the multiresponse processes was achieved through Derringer's desirability function with the assistance of a full factorial design. Separation was achieved using a C18 Phenomenex Luna column (250 x 4.6 mm id, 5 microm particle size) at a flow rate of 0.8 mL/min at 210 nm. The optimized mobile phase composition was ammonium-acetate buffer (pH 5) in pump A and acetonitrile-methanol (in equal ratio) in pump B with a run time of 40 min using a gradient method.
Impact of ACE2 gene polymorphism on antihypertensive efficacy of ACE inhibitors.[Pubmed:27121444]
J Hum Hypertens. 2016 Dec;30(12):766-771.
Angiotensin-converting enzyme 2 (ACE2), a newly discovered member of renin-angiotensin-aldosterone system, counterbalances the actions of angiotensin-converting enzyme. The objective of our study was to assess the association between rs2106809 polymorphism in ACE2 gene and the blood pressure response to ACE inhibitors in untreated hypertensive patients. After a 2-week, double-blind placebo run-in period, either benazepril or imidapril was administered for 6 weeks to 497 patients with mild to moderate essential hypertension. The achieved changes in BP were analyzed for their association with genotypes at ACE2 gene loci. In female hypertensive patients, the genotype frequency of ACE2 rs2106809 was 36.7%, 45.2% and 18.1% for CC, CT and TT genotypes, respectively. After 6 weeks of treatment, the reductions in diastolic blood pressure were significantly greater in female patients carrying the CC or CT genotype compared with those carrying the TT genotype (9.62+/-6.83 or 10.2+/-7.2 versus 6.81+/-6.31 mm Hg, respectively; P=0.045, analysis of variance (ANOVA)). Moreover, the reductions in mean arterial pressure were significantly greater in female patients carrying the CC or CT genotype compared with those carrying the TT genotype (12.1+/-7.5 or 12.0+/-7.9 versus 8.38+/-6.83 mm Hg, respectively; P=0.035, ANOVA). In male hypertensive patients, the genotype frequency of ACE2 rs2106809 was 58.1% and 41.9% for C and T genotypes, respectively. However, no association could be observed in males. We conclude that ACE2 rs2106809 is an important predictive factor of the response to antihypertensive treatment with ACE inhibitors in Chinese female hypertensive patients.
Oral Administration of N-Acetyl-seryl-aspartyl-lysyl-proline Ameliorates Kidney Disease in Both Type 1 and Type 2 Diabetic Mice via a Therapeutic Regimen.[Pubmed:27088094]
Biomed Res Int. 2016;2016:9172157.
Kidney fibrosis is the final common pathway of progressive kidney diseases including diabetic nephropathy. Here, we report that the endogenous antifibrotic peptide N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP), the substrate of angiotensin-converting enzyme (ACE), is an orally available peptide drug used to cure kidney fibrosis in diabetic mice. We utilized two mouse models of diabetic nephropathy, streptozotocin- (STZ-) induced type 1 diabetic CD-1 mice and type 2 diabetic nephropathy model db/db mice. Intervention with the ACE inhibitor imidapril, oral AcSDKP, or imidapril + oral AcSDKP combination therapy increased urine AcSDKP levels. AcSDKP levels were significantly higher in the combination group compared to those of the other groups. AcSDKP oral administration, either AcSDKP alone or in addition to imidapril, ameliorated glomerulosclerosis and tubulointerstitial fibrosis. Plasma cystatin C levels were higher in both models, at euthanasia, and were restored by all the treatment groups. The levels of antifibrotic miRs, such as miR-29 or let-7, were suppressed in the kidneys of both models; all treatments, especially the combination of imidapril + oral AcSDKP, restored the antifibrotic miR levels to a normal value or even higher. AcSDKP may be an oral antifibrotic peptide drug that would be relevant to combating fibroproliferative kidney diseases such as diabetic nephropathy.


