LDN 212320CAS# 894002-50-7 |
- ASP3026
Catalog No.:BCC1372
CAS No.:1097917-15-1
- CH5424802
Catalog No.:BCC3749
CAS No.:1256580-46-7
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- ALK inhibitor 1
Catalog No.:BCC1339
CAS No.:761436-81-1
- ALK inhibitor 2
Catalog No.:BCC1340
CAS No.:761438-38-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 894002-50-7 | SDF | Download SDF |
| PubChem ID | 7207425 | Appearance | Powder |
| Formula | C17H15N3S | M.Wt | 293.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LDN/OSU-0212320; LDN-0212320; OSU-0212320 | ||
| Solubility | DMSO : 50 mg/mL (170.42 mM; Need ultrasonic) | ||
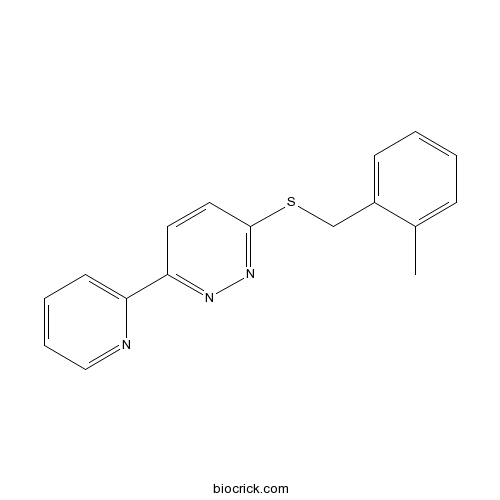
| Chemical Name | 3-[(2-methylphenyl)methylsulfanyl]-6-pyridin-2-ylpyridazine | ||
| SMILES | CC1=CC=CC=C1CSC2=NN=C(C=C2)C3=CC=CC=N3 | ||
| Standard InChIKey | DUUQLWDHNYFUPP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H15N3S/c1-13-6-2-3-7-14(13)12-21-17-10-9-16(19-20-17)15-8-4-5-11-18-15/h2-11H,12H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Increases expression of glutamate transporter EAAT2 in PA-EAAT2 cells. Displays neuroprotective activity in vivo. Shown to improve learning and memory, restore synaptic integrity and reduce amyloid plaque burden in APPSw/Ind mice. |

LDN 212320 Dilution Calculator

LDN 212320 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4084 mL | 17.0422 mL | 34.0843 mL | 68.1686 mL | 85.2108 mL |
| 5 mM | 0.6817 mL | 3.4084 mL | 6.8169 mL | 13.6337 mL | 17.0422 mL |
| 10 mM | 0.3408 mL | 1.7042 mL | 3.4084 mL | 6.8169 mL | 8.5211 mL |
| 50 mM | 0.0682 mL | 0.3408 mL | 0.6817 mL | 1.3634 mL | 1.7042 mL |
| 100 mM | 0.0341 mL | 0.1704 mL | 0.3408 mL | 0.6817 mL | 0.8521 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LDN-212320(OSU-0212320) is a glutamate transporter EAAT2 activator; enhances EAAT2 levels by > 6 fold at concentrations < 5 μM after 24 h. IC50 value: 1.83 uM(EC50) [2] Target: EAAT2 activator in vitro: LDN/OSU-0212320 increased EAAT2 protein levels in a dose-dependent(EC50=1.83 ± 0.27 μM) and time-dependent manner. LDN/OSU-0212320 increased EAAT2 protein levels and glutamate uptake function, but did not affect EAAT1 or EAAT3 protein levels. LDN/OSU-0212320 treatment markedly prevented neuronal loss and degeneration, as assessed by MAP2 immunostaining [2]. in vivo: After a single i.p. 40-mg/kg dose of LDN/OSU-0212320, EAAT2 protein levels and associated glutamate uptake increased by approximately 1.5- to 2-fold at 2 hours and by approximately 2- to 3-fold between 8 and 24 hours after injection. Even 72 hours after injection, an approximately 1.5-fold increase in EAAT2 protein levels could still be detected (data not shown). In addition, we found that LDN/OSU-0212320–induced EAAT2 protein levels and glutamate uptake were dose dependent [2]. Small-molecule activator of glutamate transporter EAAT2 translation provides neuroprotection By Kong, Qiongman; Chang, Ling-Chu; Takahashi, Kou; Liu, Qibing; Schulte, Delanie A.; Lai, Liching; Ibabao, Brian; Lin, Yuchen; Stouffer, Nathan; Das Mukhopadhyay, Chitra; et al From Journal of Clinical Investigation (2014), 124(3), 1255-1267. Structure-activity relationship study of pyridazine derivatives as glutamate transporter EAAT2 activators By Xing, Xuechao; Chang, Ling-Chu; Kong, Qiongman; Colton, Craig K.; Lai, Liching; Glicksman, Marcie A.; Lin, Chien-Liang Glenn; Cuny, Gregory D. From Bioorganic & Medicinal Chemistry Letters (2011), 21(19), 5774-5777.
References:
[1]. Xing X, et al. Structure-activity relationship study of pyridazine derivatives as glutamate transporter EAAT2 activators. Bioorg Med Chem Lett. 2011 Oct 1;21(19):5774-7.
[2]. Kong Q, et al. Small-molecule activator of glutamate transporter EAAT2 translation provides neuroprotection. J Clin Invest. 2014 Mar;124(3):1255-67.
- VU 0240551
Catalog No.:BCC5424
CAS No.:893990-34-6
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
- PF 945863
Catalog No.:BCC6172
CAS No.:893556-85-9
- Riligustilide
Catalog No.:BCC9136
CAS No.:89354-45-0
- Chiisanoside
Catalog No.:BCN2712
CAS No.:89354-01-8
- ICI 174,864
Catalog No.:BCC5675
CAS No.:89352-67-0
- 3,4-O,O-Methylene-(+)-catechin
Catalog No.:BCN7962
CAS No.:89329-14-6
- 2,4-Dihydroxy-3-nitropyridine
Catalog No.:BCC8499
CAS No.:89282-12-2
- MF63
Catalog No.:BCC1744
CAS No.:892549-43-8
- 2-(Chloromethyl)-4-(4-nitrophenyl)-1,3-thiazole
Catalog No.:BCC8372
CAS No.:89250-26-0
- AZD 3988
Catalog No.:BCC5621
CAS No.:892489-52-0
- Manidipine 2HCl
Catalog No.:BCC4405
CAS No.:89226-75-5
- Raddeanin A
Catalog No.:BCN1084
CAS No.:89412-79-3
- STF-118804
Catalog No.:BCC4850
CAS No.:894187-61-2
- [D-Trp7,9,10]-Substance P
Catalog No.:BCC7202
CAS No.:89430-38-6
- DMOG
Catalog No.:BCC2433
CAS No.:89464-63-1
- BIBF 1202
Catalog No.:BCC5298
CAS No.:894783-71-2
- ST 2825
Catalog No.:BCC1967
CAS No.:894787-30-5
- TCS JNK 6o
Catalog No.:BCC7607
CAS No.:894804-07-0
- Picrasinol B
Catalog No.:BCN4440
CAS No.:89498-91-9
- Methylneoquassin
Catalog No.:BCN3121
CAS No.:89498-93-1
- SC 144
Catalog No.:BCC1171
CAS No.:895158-95-9
- Flumatinib mesylate
Catalog No.:BCC3970
CAS No.:895519-91-2
- Mogroside IV
Catalog No.:BCN2532
CAS No.:89590-95-4
Functional modulation on macrophage by low dose naltrexone (LDN).[Pubmed:27561742]
Int Immunopharmacol. 2016 Oct;39:397-402.
Previously it was confirmed that naltrexone, a non-peptide delta-opioid receptor selective antagonist is mainly used for alcoholic dependence and opioid addiction treatment. However, there is increasing data on immune regulation of low dose naltrexone (LDN). The aim of this work was to explore the effect of LDN on the phenotype and function of macrophage. The changes of macrophage after treatment with LDN were examined using flow cytometry (FCM); FITC-dextran phagocytosis and enzyme-linked immunosorbent assay (ELISA). We have found that LDN enhances function of macrophage as confirmed by up-regulating MHC II molecule and CD64 on macrophage while down-regulating CD206 expression. Furthermore the productions of TNF-alpha, IL-6, IL-1beta, increased significantly. Macrophages in LDN treated group performed the enhanced phagocytosis. Therefore it is concluded that LDN could promote function of macrophage and this work has provided concrete data of impact on immune system by LDN. Especially the data would support interaction between CD4+T cell and macrophage in AIDS treatment with LDN in Africa (LDN has already been approved in Nigeria for the use in AIDS treatment).
A sudden and unprecedented increase in low dose naltrexone (LDN) prescribing in Norway. Patient and prescriber characteristics, and dispense patterns. A drug utilization cohort study.[Pubmed:27670755]
Pharmacoepidemiol Drug Saf. 2017 Feb;26(2):136-142.
PURPOSE: Following a TV documentary in 2013, there was a tremendous increase in low dose naltrexone (LDN) use in a wide range of unapproved indications in Norway. We aim to describe the extent of this sudden and unprecedented increase in LDN prescribing, to characterize patients and LDN prescribers, and to estimate LDN dose sizes. METHODS: LDN prescriptions recorded in the Norwegian Prescription Database (NorPD) in 2013 and 2014, and sales data not recorded in NorPD from the only Norwegian LDN manufacturer were included in the study. RESULTS: According to NorPD, 15 297 patients (0.3% of population) collected at least one LDN prescription. The actual number of users was higher as at least 23% of total sales were not recorded in NorPD. After an initial wave, there was a steady stream of new and persistent users throughout the study period. Median patient age was 52 years, and 74% of patients were female. Median daily dose was 3.7 mg. Twenty percent of all doctors and 71% of general medicine practitioners registered in Norway in 2014 prescribed LDN at least once. CONCLUSIONS: The TV documentary on LDN in Norway was followed by a large increase in LDN prescribing, and the proportion of LDN users went from an insignificant number to 0.3% of the population. There was a high willingness to use and prescribe off label despite limited evidence. Observed median LDN dose, and age and gender distribution were as expected in typical LDN using patients. (c) 2016 The Authors. Pharmacoepidemiology and Drug Safety Published by John Wiley & Sons Ltd.


