Flumatinib mesylateCAS# 895519-91-2 |
- Tyrphostin AG 1296
Catalog No.:BCC1195
CAS No.:146535-11-7
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- Pazopanib (GW-786034)
Catalog No.:BCC1286
CAS No.:444731-52-6
- Masitinib (AB1010)
Catalog No.:BCC1260
CAS No.:790299-79-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 895519-91-2 | SDF | Download SDF |
| PubChem ID | 46910592 | Appearance | Powder |
| Formula | C30H33F3N8O4S | M.Wt | 658.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 50 mg/mL (75.91 mM; Need ultrasonic) DMSO : 50 mg/mL (75.91 mM; Need ultrasonic) | ||
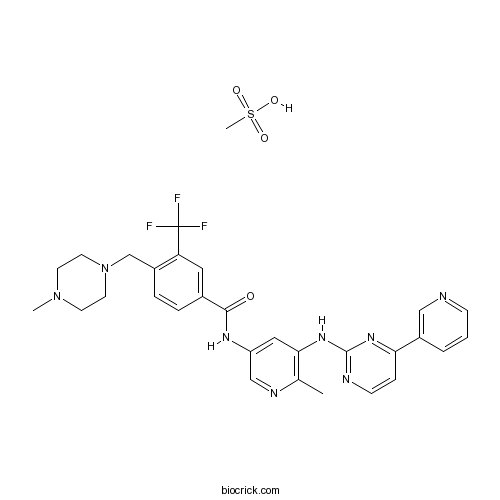
| Chemical Name | methanesulfonic acid;4-[(4-methylpiperazin-1-yl)methyl]-N-[6-methyl-5-[(4-pyridin-3-ylpyrimidin-2-yl)amino]pyridin-3-yl]-3-(trifluoromethyl)benzamide | ||
| SMILES | CC1=C(C=C(C=N1)NC(=O)C2=CC(=C(C=C2)CN3CCN(CC3)C)C(F)(F)F)NC4=NC=CC(=N4)C5=CN=CC=C5.CS(=O)(=O)O | ||
| Standard InChIKey | ZSASDYCFROUKTJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H29F3N8O.CH4O3S/c1-19-26(38-28-34-9-7-25(37-28)21-4-3-8-33-16-21)15-23(17-35-19)36-27(41)20-5-6-22(24(14-20)29(30,31)32)18-40-12-10-39(2)11-13-40;1-5(2,3)4/h3-9,14-17H,10-13,18H2,1-2H3,(H,36,41)(H,34,37,38);1H3,(H,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Flumatinib (HH-GV-678) ,a derivative of imatinib, is a multi-kinase inhibitor with IC50 Values of 1.2 nM, 307.6 nM and 2662 nM for c-Abl, PDGFRβ and c-Kit respectively. |

Flumatinib mesylate Dilution Calculator

Flumatinib mesylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5182 mL | 7.5908 mL | 15.1816 mL | 30.3633 mL | 37.9541 mL |
| 5 mM | 0.3036 mL | 1.5182 mL | 3.0363 mL | 6.0727 mL | 7.5908 mL |
| 10 mM | 0.1518 mL | 0.7591 mL | 1.5182 mL | 3.0363 mL | 3.7954 mL |
| 50 mM | 0.0304 mL | 0.1518 mL | 0.3036 mL | 0.6073 mL | 0.7591 mL |
| 100 mM | 0.0152 mL | 0.0759 mL | 0.1518 mL | 0.3036 mL | 0.3795 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Flumatinib mesylate (HH-GV-678 mesylate), a derivative of imatinib, is a multi-kinase inhibitor with IC50 Values of 1.2 nM, 307.6 nM and 2662 nM for c-Abl, PDGFRβ and c-Kit respectively. IC50 Value: 1.2 nM (c-Abl); 307.6 nM(PDGFRβ); 2662 nM (c-Kit) [1] Target: c-Abl; c-Kit; PDGRFβ in vitro: HH-GV-678 can predominantly inhibit the autophosphorylation of Bcr-Abl in K562 cell. In higher concentration, HH-GV-678 can inhibit the phosphorylation of c-Kit in Mo7e cell and the phosphorylation of PDGFR in Swiss3T3 cell, however, HH-GV-678 has no or little effect on other tyrosine kinase including EGFR、KDR、c-Src andHER2 [1]. Flumatinib effectively overcame the drug resistance of certain KIT mutants with activation loop mutations (i.e., D820G, N822K, Y823D, and A829P) [2]. in vivo: The purpose of this study was to identify the metabolites of flumatinib in CML patients, with the aim of determining the main metabolic pathways offlumatinib in humans after oral administration. Ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry revealed 34 metabolites; 7 primary metabolites were confirmed by comparison with synthetic reference standards. The results show that the parent drugflumatinib was the main form recovered in human plasma, urine, and feces. The main metabolites of flumatinib in humans were the products of N-demethylation, N-oxidation, hydroxylation, and amide hydrolysis [3].
References:
[1]. Luo H, et al. HH-GV-678, a novel selective inhibitor of Bcr-Abl, outperforms imatinib and effectively overrides imatinib resistance. Leukemia. 2010 Oct;24(10):1807-9.
[2]. Zhao J, et al. Flumatinib, a selective inhibitor of BCR-ABL/PDGFR/KIT, effectively overcomes drug resistance of certain KIT mutants. Cancer Sci. 2013 Nov 10.
[3]. Gong A, et al. Metabolism of flumatinib, a novel antineoplastic tyrosine kinase inhibitor, in chronic myelogenous leukemia patients. Drug Metab Dispos. 2010 Aug;38(8):1328-40.
- SC 144
Catalog No.:BCC1171
CAS No.:895158-95-9
- Methylneoquassin
Catalog No.:BCN3121
CAS No.:89498-93-1
- Picrasinol B
Catalog No.:BCN4440
CAS No.:89498-91-9
- TCS JNK 6o
Catalog No.:BCC7607
CAS No.:894804-07-0
- ST 2825
Catalog No.:BCC1967
CAS No.:894787-30-5
- BIBF 1202
Catalog No.:BCC5298
CAS No.:894783-71-2
- DMOG
Catalog No.:BCC2433
CAS No.:89464-63-1
- [D-Trp7,9,10]-Substance P
Catalog No.:BCC7202
CAS No.:89430-38-6
- STF-118804
Catalog No.:BCC4850
CAS No.:894187-61-2
- Raddeanin A
Catalog No.:BCN1084
CAS No.:89412-79-3
- LDN 212320
Catalog No.:BCC6361
CAS No.:894002-50-7
- VU 0240551
Catalog No.:BCC5424
CAS No.:893990-34-6
- Mogroside IV
Catalog No.:BCN2532
CAS No.:89590-95-4
- Mogroside VI
Catalog No.:BCN2578
CAS No.:89590-98-7
- Chamaejasmenin C
Catalog No.:BCN3043
CAS No.:89595-70-0
- Chamaejasmenin A
Catalog No.:BCN3044
CAS No.:89595-71-1
- 2-Acetamidoethyl phosphate
Catalog No.:BCN1760
CAS No.:89603-45-2
- Imiquimod maleate
Catalog No.:BCC4197
CAS No.:896106-16-4
- trans-3-Oxo-alpha-ionol
Catalog No.:BCN3385
CAS No.:896107-70-3
- AT9283
Catalog No.:BCC2173
CAS No.:896466-04-9
- BMH-21
Catalog No.:BCC5580
CAS No.:896705-16-1
- VX-11e
Catalog No.:BCC2051
CAS No.:896720-20-0
- Androstadienedione
Catalog No.:BCC8824
CAS No.:897-06-3
- KX2-391
Catalog No.:BCC5080
CAS No.:897016-82-9
[Effect of flumatinib mesylate on C-MYC, HIF-1alpha and VEGF in U226 line].[Pubmed:24370036]
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013 Dec;21(6):1496-500.
The objective of this study was to investigate the effect of the new generation of tyrosine kinase inhibitor Flumatinib mesylate on C-MYC, HIF-1alpha and VEGF in multiple myeloma (MM) cell line U266. Different concentrations (1, 5, 10 micromol/L) of Flumatinib mesylate were used to act on U266 cell line for 8, 16 and 24 h, and the expression of C-MYC, and HIF-1alpha genes was detected by real-time fluorescence-quantitative PCR, the expression of C-MYC, HIF-1alpha and VEGF was measured by Western blot. The results showed that the gene expression of C-MYC and HIF-1 genes decreased gradually with the increasing of Flumatinib mesylate concentration (P < 0.05). At the same concentration of Flumatinib mesylate, the expression of C-MYC and HIF-1alpha gene decreased gradually with prolonging of treatment time with the Flumatinib mesylate (P < 0.05). When the Flumatinib mesylate acted the U266 cell line for 16 h, the expression of C-MYC, HIF-1alpha and VEGF decreased gradually with the increasing of Flumatinib mesylate concentration (P < 0.05). It is concluded that the Flumatinib mesylate can reduce the expression of C-MYC, HIF-1 alpha and VEGF in U266 cell line in a time- and dose-dependent manners, so Flumatinib mesylate may become a new drug for MM therapy.


