TCS JNK 6oJNK inhibitor CAS# 894804-07-0 |
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- DL-AP3
Catalog No.:BCC2459
CAS No.:20263-06-3
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 894804-07-0 | SDF | Download SDF |
| PubChem ID | 11624601 | Appearance | Powder |
| Formula | C18H20N4O4 | M.Wt | 356.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
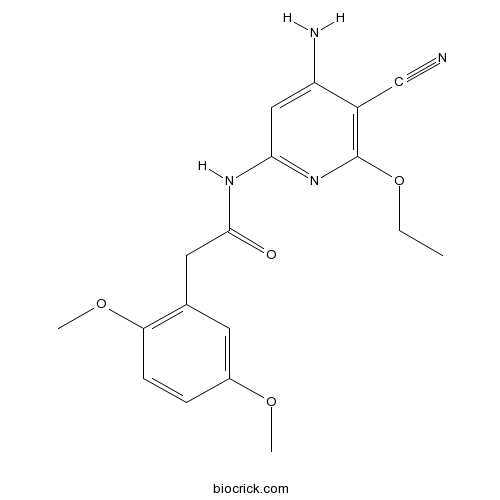
| Chemical Name | N-(4-amino-5-cyano-6-ethoxypyridin-2-yl)-2-(2,5-dimethoxyphenyl)acetamide | ||
| SMILES | CCOC1=C(C(=CC(=N1)NC(=O)CC2=C(C=CC(=C2)OC)OC)N)C#N | ||
| Standard InChIKey | KQMPRSZTUSSXND-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H20N4O4/c1-4-26-18-13(10-19)14(20)9-16(22-18)21-17(23)8-11-7-12(24-2)5-6-15(11)25-3/h5-7,9H,4,8H2,1-3H3,(H3,20,21,22,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | ATP-competitive c-Jun N-terminal kinase (JNK) inhibitor (IC50 values are 2, 4 and 52 nM for JNK1, JNK2 and JNK3 respectively). Displays > 1000 fold selectivity over other kinases, including ERK2 and p38. Inhibits c-Jun phosphorylation (EC50 = 920 nM) and prevents collagen-induced platelet aggregation in vitro. |

TCS JNK 6o Dilution Calculator

TCS JNK 6o Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.806 mL | 14.03 mL | 28.0599 mL | 56.1199 mL | 70.1498 mL |
| 5 mM | 0.5612 mL | 2.806 mL | 5.612 mL | 11.224 mL | 14.03 mL |
| 10 mM | 0.2806 mL | 1.403 mL | 2.806 mL | 5.612 mL | 7.015 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1224 mL | 1.403 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5612 mL | 0.7015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 45 nM for JNK1 and 160 nM for JNK2 [1]
TCS JNK 6o is a ATP-competitive and selective c-Jun N-terminal kinase (JNK) inhibitor. JNKs belong to the mitogen-activated protein kinase (MAP kinase) family, and are responsive to stress stimuli, including ultraviolet irradiation, cytokines, osmotic shock and heat shock. JNKs also play a role in the cellular apoptosis and T cell differentiation pathway.
In vitro: CS JNK 6o, in a dose-dependent manner, inhibits phosphorylation of c-jun (EC50 = 920 nM) and prevents collagen-induced platelet aggregation. At low collagen concentrations (0.2 and 0.5 μg/ml), platelet aggregation was totally or partially impaired by 10 μM CS JNK 6o, whereas at a high collagen concentration (5 μg/ml), TCS JNK 6o had no effect [2].
In vivo:. Pharmacokinetic profiles were studied for TCS JNK 6o in Sprague-Dawley rats. TCS JNK 6o showed a short half-life of about 1 hour, with barely measurable bioavailability and rapid clearance. Microsomal incubation studies revealed that the oxidative metabolism of TCS JNK 6o was very rapid [1]
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Szczepankiewicz BG1, Kosogof C, Nelson LT, Liu G, Liu B, Zhao H, Serby MD, Xin Z, Liu M, Gum RJ, Haasch DL, Wang S, Clampit JE, Johnson EF, Lubben TH, Stashko MA, Olejniczak ET, Sun C, Dorwin SA, Haskins K, Abad-Zapatero C, Fry EH, Hutchins CW, Sham HL, Rondinone CM, Trevillyan JM. Aminopyridine-based c-Jun N-terminal kinase inhibitors with cellular activity and minimal cross-kinase activity. J Med Chem. 2006 Jun 15;49(12):3563-80.
[2] Kauskot A, Adam F, Mazharian A, Ajzenberg N, Berrou E, Bonnefoy A, Rosa JP, Hoylaerts MF, Bryckaert M. Involvement of the mitogen-activated protein kinase c-Jun NH2-terminal kinase 1 in thrombus formation. J Biol Chem. 2007 Nov 2;282(44):31990-9.
- ST 2825
Catalog No.:BCC1967
CAS No.:894787-30-5
- BIBF 1202
Catalog No.:BCC5298
CAS No.:894783-71-2
- DMOG
Catalog No.:BCC2433
CAS No.:89464-63-1
- [D-Trp7,9,10]-Substance P
Catalog No.:BCC7202
CAS No.:89430-38-6
- STF-118804
Catalog No.:BCC4850
CAS No.:894187-61-2
- Raddeanin A
Catalog No.:BCN1084
CAS No.:89412-79-3
- LDN 212320
Catalog No.:BCC6361
CAS No.:894002-50-7
- VU 0240551
Catalog No.:BCC5424
CAS No.:893990-34-6
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
- PF 945863
Catalog No.:BCC6172
CAS No.:893556-85-9
- Riligustilide
Catalog No.:BCC9136
CAS No.:89354-45-0
- Chiisanoside
Catalog No.:BCN2712
CAS No.:89354-01-8
- Picrasinol B
Catalog No.:BCN4440
CAS No.:89498-91-9
- Methylneoquassin
Catalog No.:BCN3121
CAS No.:89498-93-1
- SC 144
Catalog No.:BCC1171
CAS No.:895158-95-9
- Flumatinib mesylate
Catalog No.:BCC3970
CAS No.:895519-91-2
- Mogroside IV
Catalog No.:BCN2532
CAS No.:89590-95-4
- Mogroside VI
Catalog No.:BCN2578
CAS No.:89590-98-7
- Chamaejasmenin C
Catalog No.:BCN3043
CAS No.:89595-70-0
- Chamaejasmenin A
Catalog No.:BCN3044
CAS No.:89595-71-1
- 2-Acetamidoethyl phosphate
Catalog No.:BCN1760
CAS No.:89603-45-2
- Imiquimod maleate
Catalog No.:BCC4197
CAS No.:896106-16-4
- trans-3-Oxo-alpha-ionol
Catalog No.:BCN3385
CAS No.:896107-70-3
- AT9283
Catalog No.:BCC2173
CAS No.:896466-04-9
Dual functions for WNT5A during cartilage development and in disease.[Pubmed:23474397]
Matrix Biol. 2013 Jun 24;32(5):252-64.
Mouse and human genetic data suggests that Wnt5a is required for jaw development but the specific role in facial skeletogenesis is unknown. We mapped expression of WNT5A in the developing chicken skull and found that the highest expression was in early Meckel's cartilage but by stage 35 expression was decreased to background. We focused on chondrogenesis by targeting a retrovirus expressing WNT5A to the mandibular prominence prior to cell differentiation. Unexpectedly, there were no phenotypes in the first 6days following injection; however later the mandibular bones and Meckel's cartilage were reduced or missing on the treated side. To examine the effects on cartilage differentiation we treated micromass cultures from mandibular mesenchyme with Wnt5a-conditioned media (CM). Similar to in vivo viral data, cartilage differentiates normally, but, after 6days of culture, nearly all Alcian blue staining is lost. Collagen II and aggrecan were also decreased in treated cultures. The matrix loss was correlated with upregulation of metalloproteinases, MMP1, MMP13, and ADAMTS5 (codes for Aggrecanase). Moreover, Marimastat, an MMP and Aggrecanase inhibitor rescued cartilage matrix in Wnt5a-CM treated cultures. The pathways mediating these cartilage and RNA changes were investigated using luciferase assays. Wnt5a-CM was a potent inhibitor of the canonical pathway and strongly activated JNK/PCP signaling. To determine whether the matrix loss is mediated by repression of canonical signaling or activation of the JNK pathway we treated mandibular cultures with either DKK1, an antagonist of the canonical pathway, or a small molecule that antagonizes JNK signaling (TCS JNK 6o). DKK1 slightly increased cartilage formation and therefore suggested that the endogenous canonical signaling represses chondrogenesis. To test this further we added an excess of Wnt3a-CM and found that far fewer cartilage nodules differentiated. Since DKK1 did not mimic the effects of Wnt5a we excluded the canonical pathway from mediating the matrix loss phenotype. The JNK antagonist partially rescued the Wnt5a phenotype supporting this non-canonical pathway as the main mediator of the cartilage matrix degradation. Our study reveals two new roles for WNT5A in development and disease: 1) to repress canonical Wnt signaling in cartilage blastema in order to promote normal differentiation and 2) in conditions of excess to stimulate degradation of mature cartilage matrix via non-canonical pathways.
Involvement of the mitogen-activated protein kinase c-Jun NH2-terminal kinase 1 in thrombus formation.[Pubmed:17785464]
J Biol Chem. 2007 Nov 2;282(44):31990-9.
The involvement of the mitogen-activated protein kinase c-Jun NH2-terminal kinase-1 (JNK1) has never been investigated in hemostasis and thrombosis. Using two JNK inhibitors (SP600125 and 6o), we have demonstrated that JNK1 is involved in collagen-induced platelet aggregation dependent on ADP. In these conditions, JNK1 activation requires the coordinated signaling pathways of collagen receptors (alpha2beta1 and glycoprotein (GP)VI) and ADP. In contrast, JNK1 is not required for platelet adhesion on a collagen matrix in static or blood flow conditions (300-1500 s(-1)) involving collagen receptors (alpha2beta1 and GPVI). Importantly, at 1500 s(-1), JNK1 acts on thrombus formation on a collagen matrix dependent on GPIb-von Willebrand factor (vWF) interaction but not ADP receptor activation. This is confirmed by the involvement of JNK1 in shear-induced platelet aggregation at 4000 s(-1). We also provide evidence during rolling and adhesion of platelets to vWF that platelet GPIb-vWF interaction triggers alphaIIbbeta3 activation in a JNK1-dependent manner. This was confirmed with a Glanzmann thrombastenic patient lacking alphaIIbbeta3. Finally, in vivo, JNK1 is involved in arterial but not in venular thrombosis in mice. Overall, our in vitro studies define a new role of JNK1 in thrombus formation in flowing blood that is relevant to thrombus development in vivo.
Aminopyridine-based c-Jun N-terminal kinase inhibitors with cellular activity and minimal cross-kinase activity.[Pubmed:16759099]
J Med Chem. 2006 Jun 15;49(12):3563-80.
The c-Jun N-terminal kinases (JNK-1, -2, and -3) are members of the mitogen activated protein (MAP) kinase family of enzymes. They are activated in response to certain cytokines, as well as by cellular stresses including chemotoxins, peroxides, and irradiation. They have been implicated in the pathology of a variety of different diseases with an inflammatory component including asthma, stroke, Alzheimer's disease, and type 2 diabetes mellitus. In this work, high-throughput screening identified a JNK inhibitor with an excellent kinase selectivity profile. Using X-ray crystallography and biochemical screening to guide our lead optimization, we prepared compounds with inhibitory potencies in the low-double-digit nanomolar range, activity in whole cells, and pharmacokinetics suitable for in vivo use. The new compounds were over 1,000-fold selective for JNK-1 and -2 over other MAP kinases including ERK2, p38alpha, and p38delta and showed little inhibitory activity against a panel of 74 kinases.


