SorafenibRaf kinases and tyrosine kinases inhibitor CAS# 284461-73-0 |
- B-Raf inhibitor 1 dihydrochloride
Catalog No.:BCC4183
CAS No.:1191385-19-9
- Axitinib (AG 013736)
Catalog No.:BCC3729
CAS No.:319460-85-0
- SB590885
Catalog No.:BCC4392
CAS No.:405554-55-4
- Vandetanib (ZD6474)
Catalog No.:BCC3883
CAS No.:443913-73-3
- Sorafenib Tosylate
Catalog No.:BCC3654
CAS No.:475207-59-1
- Brivanib Alaninate (BMS-582664)
Catalog No.:BCC1240
CAS No.:649735-63-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 284461-73-0 | SDF | Download SDF |
| PubChem ID | 216239 | Appearance | Powder |
| Formula | C21H16ClF3N4O3 | M.Wt | 464.83 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Bay 43-9006 | ||
| Solubility | DMSO : ≥ 45 mg/mL (96.81 mM) *"≥" means soluble, but saturation unknown. | ||
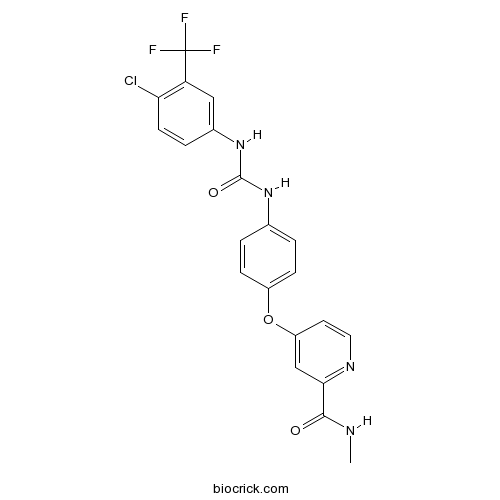
| Chemical Name | 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-N-methylpyridine-2-carboxamide | ||
| SMILES | CNC(=O)C1=NC=CC(=C1)OC2=CC=C(C=C2)NC(=O)NC3=CC(=C(C=C3)Cl)C(F)(F)F | ||
| Standard InChIKey | MLDQJTXFUGDVEO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sorafenib is a potent multikinase inhibitor with IC50s of 6 nM, 20 nM, and 22 nM for Raf-1, B-Raf, and VEGFR-3, respectively.Sorafenib together with inhibitors of the β-catenin pathway might be an effective tool in the treatment of pediatric HCC. The combination of Sorafenib with AMPK activators could have beneficial effects on tumor regression by AMPK pathway activation. The combination of metformin or other AMPK activators and Sorafenib could be tested in prospective clinical trials. |
| Targets | AMPK | Wnt/β-catenin | mTOR | ERK | VEGFR-3 | B-Raf | Raf-1 |
| In vitro | MiRNA-30a-mediated autophagy inhibition sensitizes renal cell carcinoma cells to sorafenib.[Pubmed: 25712526]Biochem Biophys Res Commun. 2015 Apr 3;459(2):234-9.Chemotherapy-induced autophagy activation often contributes to cancer resistance. MiRNA-30a (miR-30a) is a potent inhibitor of autophagy by downregulating Beclin-1. In this study, we characterized the role of miR-30a in Sorafenib-induced activity in renal cell carcinoma (RCC) cells. |
| In vivo | Cyproheptadine significantly improves the overall and progression-free survival of sorafenib-treated advanced HCC patients.[Pubmed: 25646358]Jpn J Clin Oncol. 2015 Apr;45(4):336-42.Sorafenib is a recommended treatment for advanced hepatocellular carcinoma. The study is to evaluate the efficacy of Sorafenib plus cyproheptadine compared with Sorafenib alone in patients with advanced hepatocellular carcinoma.
Biomarkers of apoptosis and necrosis in patients with hepatocellular carcinoma treated with sorafenib.[Pubmed: 25750346]Anticancer Res. 2015 Mar;35(3):1803-8.Sorafenib is the medical reference for treatment of hepatocellular carcinoma (HCC). Multiple forms of cytotoxicity are induced by Sorafenib in HCC cells in vitro but it is unclear what extent of apoptosis and necrosis is induced in HCC patients receiving Sorafenib.
|
| Kinase Assay | Sorafenib synergizes with metformin in NSCLC through AMPK pathway activation.[Pubmed: 25080865]Int J Cancer. 2015 Mar 15;136(6):1434-44.The multikinase inhibitor Sorafenib is under clinical investigation for the treatment of many solid tumors, but in most cases, the molecular target responsible for the clinical effect is unknown. Furthermore, enhancing the effectiveness of Sorafenib using combination strategies is a major clinical challenge. |
| Animal Research | Anti-tumor activity of sorafenib in a model of a pediatric hepatocellular carcinoma.[Pubmed: 25447203]Exp Cell Res. 2015 Feb 1;331(1):97-104.Treatment outcome of children with pediatric hepatocellular carcinoma (pHCC) is poor. Therefore, we evaluated the tyrosine kinase inhibitor Sorafenib in a model of pHCC.
|

Sorafenib Dilution Calculator

Sorafenib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1513 mL | 10.7566 mL | 21.5132 mL | 43.0265 mL | 53.7831 mL |
| 5 mM | 0.4303 mL | 2.1513 mL | 4.3026 mL | 8.6053 mL | 10.7566 mL |
| 10 mM | 0.2151 mL | 1.0757 mL | 2.1513 mL | 4.3026 mL | 5.3783 mL |
| 50 mM | 0.043 mL | 0.2151 mL | 0.4303 mL | 0.8605 mL | 1.0757 mL |
| 100 mM | 0.0215 mL | 0.1076 mL | 0.2151 mL | 0.4303 mL | 0.5378 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
The anti-proliferation induced by dual inhibition of PI3 K/Akt/mTOR signaling pathways and Ras/Raf/mitogen-activated protein kinase, which are associated with HCC proliferation, has been evaluated in non-liver cancer stem cell lines and LCSC lines respectively.
Abstract
Since the 200 mg tablet of sorafenib, a multikinase inhibitor with antiangiogenic and antitumor activity, is not suitable for children, extemporaneously prepared smaller-dosage of sorafenib has been investigated for stability and pharmacokinetic profile in children.
Abstract
Sorafenib is a multikinase inhibitor that exhibits antiangiogenic and antiproliferative activity and targets signaling pathways involved in liver regeneration.
Abstract
Sorafenib is a multikinase inhibitor that exerts cytotoxic effects on HCC cells through inducing ferroptosis, a iron-dependent and oxidative stress-associated cell death.
Abstract
The association between the Hand-Foot skin reaction and the clinical outcome in metastatic renal cell carcinoma patients treated with sorafenib were retrospectively examined.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sorafenib is an oral multikinase inhibitor with activity against Raf kinase and several receptor tyrosine kinases, including vascular endothelial growth factor receptor 2 (VEGFR2), platelet-derived growth factor receptor (PDGFR), FLT3, Ret, and c-Kit. sorafenib, inhibits tumor growth and disrupts tumor microvasculature through antiproliferative, antiangiogenic, and/or proapoptotic effects. sorafenib blocks Raf kinase signaling, inhibits tumor cell proliferation, and induces apoptosis in vitro. In addition, sorafenib exhibits robust antitumor efficacy.
References
1. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. L Liu, Y Cao, C Chen, X Zhang, A McNabola, D Wilkie Cancer research, 2006
2. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. GK Abou-Alfa, L Schwartz, S Ricci. Journal of clinical Oncology. 2006
- 6-Hydroxy-2,6-dimethyl-2,7-octadienoic acid
Catalog No.:BCN1463
CAS No.:28420-25-9
- Ac9-25
Catalog No.:BCC5997
CAS No.:284040-76-2
- NPS-2143
Catalog No.:BCC4409
CAS No.:284035-33-2
- DR 2313
Catalog No.:BCC2451
CAS No.:284028-90-6
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- FR 236924
Catalog No.:BCC7564
CAS No.:28399-31-7
- 1(10)-Aristolen-2-one
Catalog No.:BCN7634
CAS No.:28398-06-3
- Bumetanide
Catalog No.:BCC1119
CAS No.:28395-03-1
- 7-Neohesperidosides
Catalog No.:BCN8200
CAS No.:28383-41-7
- Aloin B
Catalog No.:BCN2576
CAS No.:28371-16-6
- Chrysin 6-C-glucoside
Catalog No.:BCN3324
CAS No.:28368-57-2
- Mahanine
Catalog No.:BCN3176
CAS No.:28360-49-8
- 4-(4-Aminophenoxy)-N-methyl-2-pyridinecarboxamide
Catalog No.:BCC8649
CAS No.:284462-37-9
- Bavachalcone
Catalog No.:BCN3193
CAS No.:28448-85-3
- Tomentin
Catalog No.:BCN5180
CAS No.:28449-62-9
- 20-Deacetyltaxuspine X
Catalog No.:BCN7374
CAS No.:284672-76-0
- 9-Deacetyltaxinine E
Catalog No.:BCN7227
CAS No.:284672-78-2
- Delphinidin-3-O-galactoside chloride
Catalog No.:BCN3019
CAS No.:28500-00-7
- Petunidin-3-O-galactoside chloride
Catalog No.:BCN3024
CAS No.:28500-02-9
- Petunidin-3-O-arabinoside chloride
Catalog No.:BCN3026
CAS No.:28500-03-0
- Malvidin-3-O-arabinoside chloride
Catalog No.:BCN3032
CAS No.:28500-04-1
- Anadoline N-oxide
Catalog No.:BCN2029
CAS No.:28513-29-3
- 1-Amino-2-methylpropan-2-ol
Catalog No.:BCN1773
CAS No.:2854-16-2
- BML-190
Catalog No.:BCC4410
CAS No.:2854-32-2
Sorafenib synergizes with metformin in NSCLC through AMPK pathway activation.[Pubmed:25080865]
Int J Cancer. 2015 Mar 15;136(6):1434-44.
The multikinase inhibitor Sorafenib is under clinical investigation for the treatment of many solid tumors, but in most cases, the molecular target responsible for the clinical effect is unknown. Furthermore, enhancing the effectiveness of Sorafenib using combination strategies is a major clinical challenge. Here, we identify Sorafenib as an activator of AMP-activated protein kinase (AMPK), in a manner that involves either upstream LKB1 or CAMKK2. We further show in a phase II clinical trial in KRAS mutant advanced non-small cell lung cancer (NSCLC) with single agent Sorafenib an improved disease control rate in patients using the antidiabetic drug metformin. Consistent with this, Sorafenib and metformin act synergistically in inhibiting cellular proliferation in NSCLC in vitro and in vivo. A synergistic effect of both drugs is also seen on phosphorylation of the AMPKalpha activation site. Our results provide a rationale for the synergistic antiproliferative effects, given that AMPK inhibits downstream mTOR signaling. These data suggest that the combination of Sorafenib with AMPK activators could have beneficial effects on tumor regression by AMPK pathway activation. The combination of metformin or other AMPK activators and Sorafenib could be tested in prospective clinical trials.
Biomarkers of apoptosis and necrosis in patients with hepatocellular carcinoma treated with sorafenib.[Pubmed:25750346]
Anticancer Res. 2015 Mar;35(3):1803-8.
BACKGROUND/AIM: Sorafenib is the medical reference for treatment of hepatocellular carcinoma (HCC). Multiple forms of cytotoxicity are induced by Sorafenib in HCC cells in vitro but it is unclear what extent of apoptosis and necrosis is induced in HCC patients receiving Sorafenib. PATIENTS AND METHODS: The M30 and M65 biomarkers, which reflect the release of cytokeratin-18 and its apoptotic cleavage fragments, were measured in patients with HCC (n=36) and matched patients with cirrhosis (n=47). A serum sample was collected from 20 patients with HCC four weeks after the onset of treatment with Sorafenib. RESULTS: Basal serum levels of M30 and M65 were increased in patients with HCC compared to those with uncomplicated cirrhosis. No statistically significant increase in the level of M30 or M65 was found in the sera of patients with HCC after Sorafenib. CONCLUSION: The findings indicate that Sorafenib is not a potent inducer of HCC cell death in most patients.
Cyproheptadine significantly improves the overall and progression-free survival of sorafenib-treated advanced HCC patients.[Pubmed:25646358]
Jpn J Clin Oncol. 2015 Apr;45(4):336-42.
OBJECTIVE: Sorafenib is a recommended treatment for advanced hepatocellular carcinoma. The study is to evaluate the efficacy of Sorafenib plus cyproheptadine compared with Sorafenib alone in patients with advanced hepatocellular carcinoma. METHODS: A retrospective cohort study reviewed all consecutive advanced hepatocellular carcinoma cases with Child-Pugh Class A disease starting Sorafenib treatment at our hospital from August 2012 to March 2013. They were followed up until 31 December 2013. A total of 52 patients were enrolled: 32 patients in the combination (Sorafenib-cyproheptadine) group and 20 patients in the control (Sorafenib alone) group. The response to treatment, overall survival and progression-free survival were compared. RESULTS: The median overall survival was 11.0 months (95% confidence interval: 6.8-15.1 months) in the combination group compared with 4.8 months (95% confidence interval: 3.1-6.6 months) in the control group (crude hazard ratio = 0.45, 95% confidence interval: 0.22-0.82). The median progression-free survival time was 7.5 months (95% confidence interval: 5.1-10.0 months) in the combination group compared with 1.7 months (95% confidence interval: 1.4-2.1 months) in the control group (crude hazard ratio = 0.43, 95% confidence interval: 0.22-0.86). Kaplan-Meier survival analysis revealed that both overall survival and progression-free survival in the combination group were significantly longer than that in the control group. The multivariate model found patients in the combination group were 76% less likely to die (adjusted hazard ratio = 0.24, 95% confidence interval: 0.10-0.58) and 82% less likely to have progression (adjusted hazard ratio = 0.18, 95% confidence interval: 0.08-0.44) during the 17 months of follow-up. CONCLUSION: Cyproheptadine may significantly improve survival outcomes of Sorafenib-treated advanced hepatocellular carcinoma patients.
MiRNA-30a-mediated autophagy inhibition sensitizes renal cell carcinoma cells to sorafenib.[Pubmed:25712526]
Biochem Biophys Res Commun. 2015 Apr 3;459(2):234-239.
Chemotherapy-induced autophagy activation often contributes to cancer resistance. MiRNA-30a (miR-30a) is a potent inhibitor of autophagy by downregulating Beclin-1. In this study, we characterized the role of miR-30a in Sorafenib-induced activity in renal cell carcinoma (RCC) cells. We found that expression of miR-30a was significantly downregulated in several human RCC tissues and in RCC cell lines. Accordingly, its targeted gene Beclin-1 was upregulated. Sorafenib activated autophagy in RCC cells (786-0 and A489 lines), evidenced by p62 degradation, Beclin-1/autophagy protein 5 (ATG-5) upregulation and light chain (LC)3B-I/-II conversion. Exogenously expressing miR-30a in 786-0 or A489 cells inhibited Beclin-1 expression and enhanced Sorafenib-induced cytotoxicity. In contrast, knockdown of miR-30a by introducing antagomiR-30a increased Beclin-1 expression, and inhibited Sorafenib-induced cytotoxicity against RCC cells. Autophagy inhibitors, including chloroquine, 3-methyaldenine or Bafliomycin A1, enhanced Sorafenib activity, causing substantial cell apoptosis. Meanwhile, knockdown of Beclin-1 or ATG-5 by targeted siRNAs also increased Sorafenib-induced cytotoxicity in above RCC cells. These findings indicate that dysregulation of miR-30a in RCC may interfere with the effectiveness of Sorafenib-mediated apoptosis by an autophagy-dependent pathway, thus representing a novel potential therapeutic target for RCC.
Anti-tumor activity of sorafenib in a model of a pediatric hepatocellular carcinoma.[Pubmed:25447203]
Exp Cell Res. 2015 Feb 1;331(1):97-104.
BACKGROUND: Treatment outcome of children with pediatric hepatocellular carcinoma (pHCC) is poor. Therefore, we evaluated the tyrosine kinase inhibitor Sorafenib in a model of pHCC. METHODS: Cell viability after treatment with Sorafenib was evaluated in HC-AFW1 cells (pHCC) using MTT assay and compared to an adult HCC (aHCC) and two hepatoblastoma (HB) cell lines. ERK, pERK, E-cadherin, and vimentin expression were investigated using Western Blot. Sorafenib (60 mg/kg) was administered orally to NOD.Cg-Prkdcscid-IL2rgtmWjl/Sz mice bearing subcutaneous HC-AFW1-derived tumors. Tumor progression, viability, and vascularization were monitored by tumor volume, AFP levels, and CD31 immunostaining, respectively. Sensitization to Sorafenib was evaluated using the beta-catenin inhibitor ICG001. RESULTS: Sorafenib reduced cell viability in HC-AFW1 (IC50: 8 microM), comparable to HB cells, however less pronounced in aHCC cells (IC50: 23 microM). Sorafenib inhibited ERK signaling in both, HC-AFW1 cells and -xenografts. In vivo, Sorafenib treatment only led to a moderate tumor growth inhibition, although significant reduction of vascularization and tumor growth kinetics was observed. Long-term treatment with Sorafenib decreased E-cadherin, but showed no induction of vimentin expression. Combining Sorafenib with a beta-catenin inhibitor led to an additional reduction of cell viability. CONCLUSION: Sorafenib together with inhibitors of the beta-catenin pathway might be an effective tool in the treatment of pediatric HCC.


