TomentinCAS# 28449-62-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28449-62-9 | SDF | Download SDF |
| PubChem ID | 14059525 | Appearance | White powder |
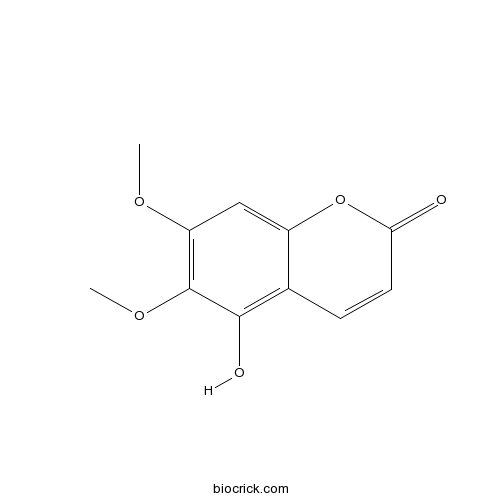
| Formula | C11H10O5 | M.Wt | 222.2 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Synonyms | 5-Hydroxy 6,7-dimethoxycoumarin | ||
| Solubility | Soluble in DMSO | ||
| Chemical Name | 5-hydroxy-6,7-dimethoxychromen-2-one | ||
| SMILES | COC1=C(C(=C2C=CC(=O)OC2=C1)O)OC | ||
| Standard InChIKey | KCPNDUHAXDHRKX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H10O5/c1-14-8-5-7-6(3-4-9(12)16-7)10(13)11(8)15-2/h3-5,13H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Tomentin has anti-inflammatory effects, it can inhibit the formation of λ-carrageenan footpad edema at 58%. |
| Targets | Immunology & Inflammation related |

Tomentin Dilution Calculator

Tomentin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5005 mL | 22.5023 mL | 45.0045 mL | 90.009 mL | 112.5113 mL |
| 5 mM | 0.9001 mL | 4.5005 mL | 9.0009 mL | 18.0018 mL | 22.5023 mL |
| 10 mM | 0.45 mL | 2.2502 mL | 4.5005 mL | 9.0009 mL | 11.2511 mL |
| 50 mM | 0.09 mL | 0.45 mL | 0.9001 mL | 1.8002 mL | 2.2502 mL |
| 100 mM | 0.045 mL | 0.225 mL | 0.45 mL | 0.9001 mL | 1.1251 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bavachalcone
Catalog No.:BCN3193
CAS No.:28448-85-3
- 4-(4-Aminophenoxy)-N-methyl-2-pyridinecarboxamide
Catalog No.:BCC8649
CAS No.:284462-37-9
- Sorafenib
Catalog No.:BCN2174
CAS No.:284461-73-0
- 6-Hydroxy-2,6-dimethyl-2,7-octadienoic acid
Catalog No.:BCN1463
CAS No.:28420-25-9
- Ac9-25
Catalog No.:BCC5997
CAS No.:284040-76-2
- NPS-2143
Catalog No.:BCC4409
CAS No.:284035-33-2
- DR 2313
Catalog No.:BCC2451
CAS No.:284028-90-6
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- FR 236924
Catalog No.:BCC7564
CAS No.:28399-31-7
- 1(10)-Aristolen-2-one
Catalog No.:BCN7634
CAS No.:28398-06-3
- Bumetanide
Catalog No.:BCC1119
CAS No.:28395-03-1
- 7-Neohesperidosides
Catalog No.:BCN8200
CAS No.:28383-41-7
- 20-Deacetyltaxuspine X
Catalog No.:BCN7374
CAS No.:284672-76-0
- 9-Deacetyltaxinine E
Catalog No.:BCN7227
CAS No.:284672-78-2
- Delphinidin-3-O-galactoside chloride
Catalog No.:BCN3019
CAS No.:28500-00-7
- Petunidin-3-O-galactoside chloride
Catalog No.:BCN3024
CAS No.:28500-02-9
- Petunidin-3-O-arabinoside chloride
Catalog No.:BCN3026
CAS No.:28500-03-0
- Malvidin-3-O-arabinoside chloride
Catalog No.:BCN3032
CAS No.:28500-04-1
- Anadoline N-oxide
Catalog No.:BCN2029
CAS No.:28513-29-3
- 1-Amino-2-methylpropan-2-ol
Catalog No.:BCN1773
CAS No.:2854-16-2
- BML-190
Catalog No.:BCC4410
CAS No.:2854-32-2
- Theaflavin-3'-gallate
Catalog No.:BCN5421
CAS No.:28543-07-9
- 4-Benzoyloxy-2-azetidinone
Catalog No.:BCC8696
CAS No.:28562-58-5
- 7-Geranyloxy-6-methoxycoumarin
Catalog No.:BCN5181
CAS No.:28587-43-1
Sphaeralcic acid and tomentin, anti-inflammatory compounds produced in cell suspension cultures of Sphaeralcea angustifolia.[Pubmed:24488717]
Planta Med. 2014 Feb;80(2-3):209-14.
Sphaeralcea angustifolia, an endangered plant species in Mexico, is employed to treat inflammatory processes and as a wound healing remedy. Scopoletin (1) was reported as one of the main bioactive compounds in this plant. Here, we isolated and identified compounds with anti-inflammatory properties from the suspension-cultured cells of S. angustifolia. The CH2Cl2 : CH3OH extract of the cells exhibited anti-inflammatory properties in acute inflammation models. Two compounds were isolated, 5-hydroxy-6,7-dimethoxycoumarin, named Tomentin (2), and 2-(1,8-dihydroxy-4-isopropyl-6-methyl-7-methoxy)-naphthoic acid, denominated as sphaeralcic acid (3). Their structures were determined by spectroscopic and spectrometric analyses. The anti-inflammatory effects of both compounds were also evaluated. At a dose of 45 mg/kg, compound 2 inhibited the formation of lambda-carrageenan footpad edema at 58 %, and compound 3 at 66 %. Local application of compound 2 (225 mM per ear) or 3 (174 mM per ear) inhibited the phorbol ester-induced auricular edema formation by 57 % or 86 %, respectively. The effect of compound 3 was dose-dependent and the ED50 was 93 mM.


