CandesartanAngiotensin II receptor 1 (AT1) antagonist CAS# 139481-59-7 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Rosuvastatin Calcium
Catalog No.:BCC3853
CAS No.:147098-20-2
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 139481-59-7 | SDF | Download SDF |
| PubChem ID | 2541 | Appearance | Powder |
| Formula | C24H20N6O3 | M.Wt | 440.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (227.04 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
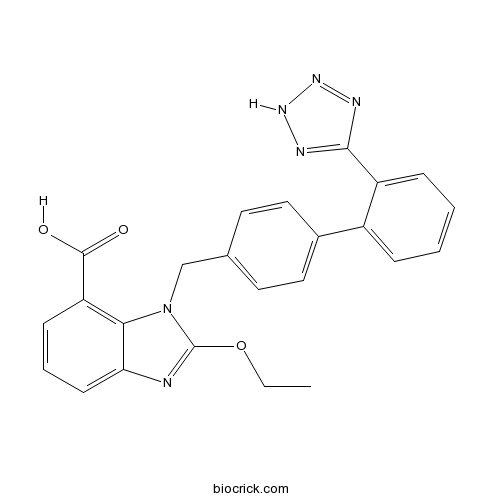
| Chemical Name | 2-ethoxy-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]benzimidazole-4-carboxylic acid | ||
| SMILES | CCOC1=NC2=CC=CC(=C2N1CC3=CC=C(C=C3)C4=CC=CC=C4C5=NNN=N5)C(=O)O | ||
| Standard InChIKey | HTQMVQVXFRQIKW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Angiotensin II receptor 1 (AT1) antagonist (IC50 values are 1.12 and 2.86 nM in bovine adrenal cortex and rabbit aorta, respectively). Exhibits antihypertensive effects in animal models. Also available as a prodrug, candesartan cilexetil. |

Candesartan Dilution Calculator

Candesartan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2704 mL | 11.352 mL | 22.7041 mL | 45.4081 mL | 56.7601 mL |
| 5 mM | 0.4541 mL | 2.2704 mL | 4.5408 mL | 9.0816 mL | 11.352 mL |
| 10 mM | 0.227 mL | 1.1352 mL | 2.2704 mL | 4.5408 mL | 5.676 mL |
| 50 mM | 0.0454 mL | 0.227 mL | 0.4541 mL | 0.9082 mL | 1.1352 mL |
| 100 mM | 0.0227 mL | 0.1135 mL | 0.227 mL | 0.4541 mL | 0.5676 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Angiotensin II receptor 1 (AT1) antagonist (IC50 values are 1.12 and 2.86 nM in bovine adrenal cortex and rabbit aorta, respectively). Exhibits antihypertensive effects in animal models. Also available as a pro-drug candesartan cilex
- Candesartan ethyl ester
Catalog No.:BCC8901
CAS No.:139481-58-6
- Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate
Catalog No.:BCC9032
CAS No.:139481-44-0
- Ethyl 2-ethoxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylate
Catalog No.:BCC8970
CAS No.:139481-41-7
- Methyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC9033
CAS No.:139481-28-0
- 6-O-apiosyl-5-O-Methylvisammioside
Catalog No.:BCN7858
CAS No.:139446-82-5
- Boc-Cysteinol(Bzl)
Catalog No.:BCC3043
CAS No.:139428-96-9
- 8alpha-Hydroxyhirsutinolide
Catalog No.:BCN7111
CAS No.:1394156-45-6
- GNE-317
Catalog No.:BCC5655
CAS No.:1394076-92-6
- Tiotropium Bromide hydrate
Catalog No.:BCC4585
CAS No.:139404-48-1
- Guan-fu base A
Catalog No.:BCN8491
CAS No.:1394-48-5
- TC LPA5 4
Catalog No.:BCC6267
CAS No.:1393814-38-4
- KPT-330
Catalog No.:BCC4446
CAS No.:1393477-72-9
- Candesartan methyl ester
Catalog No.:BCC8902
CAS No.:139481-69-9
- Trityl candesartan
Catalog No.:BCC9187
CAS No.:139481-72-4
- GSK J5
Catalog No.:BCC6264
CAS No.:1394854-51-3
- GSK J2
Catalog No.:BCC6263
CAS No.:1394854-52-4
- MS436
Catalog No.:BCC4037
CAS No.:1395084-25-9
- Buddlejasaponin IV
Catalog No.:BCN5344
CAS No.:139523-30-1
- 2-(7-Methoxy-1-naphthyl)ethylamine hydrochloride
Catalog No.:BCN1574
CAS No.:139525-77-2
- Fmoc-Leu-ol
Catalog No.:BCC2582
CAS No.:139551-83-0
- Cannabidiol
Catalog No.:BCN6208
CAS No.:13956-29-1
- Lycoclavanol
Catalog No.:BCN6209
CAS No.:13956-51-9
- Serratriol
Catalog No.:BCN6210
CAS No.:13956-52-0
- Epicannabidiol hydrate
Catalog No.:BCN6207
CAS No.:139561-95-8
Effects of Candesartan Cilexetil Compared with Amlodipine on Serum Asymmetric Dimethylarginine Levels in the Chronic Stage of Cerebral Infarction: A Preliminary Study.[Pubmed:28133009]
J Nippon Med Sch. 2016;83(6):272-276.
OBJECTIVE: Asymmetric dimethylarginine (ADMA) is an endogenous nitric oxide synthase inhibitor and a marker of vascular endothelial damage. Angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker (ARB) are reported to reduce the serum ADMA level. Our group administered either ARB or calcium antagonist to patients after cerebral infarction and discussed the ADMA changes observed. METHODS: Hypertensives in the chronic stage of cerebral infarction were enrolled. These subjects included patients of atherothrombotic cerebral infarction or lacunar infarction. The patients received Candesartan cilexetil (Candesartan group) or amlodipine (amlodipine group). The blood pressure and serum ADMA concentration were measured and compared before the treatment commenced and at 1-3 months after the treatment commenced. RESULTS: Seven subjects received Candesartan and six received amlodipine. There was no difference between the groups in the change of blood pressure before and after the drug treatment. The ADMA level (nmol/mL) fell significantly from 0.57+/-0.10 (before administration) to 0.52+/-0.09 (after administration) in the Candesartan group (P<0.05). The ADMA level did not change between before and after administration in the amlodipine group. CONCLUSION: Treatment with Candesartan cilexetil reduced the level of ADMA in hypertensive patients in the chronic stage of cerebral infarction. Candesartan cilexetil may be useful in hypertensive patients at the chronic stage of cerebral infarction with expected anti-atherosclerotic effect.
[Prevention of Cardiovascular Complications in Patients With Arterial Hypertension While Use of Angiotensin II Receptor Antagonists. Possibilities of Candesartan].[Pubmed:28290850]
Kardiologiia. 2016 Jun;56(6):63-67.
The article demonstrates advantages of angiotensin II receptor antagonists in treatment of arterial hypertension (AH). Subjects for consideration are evidences of antihypertensive efficacy of Candesartan and possibilities of the product in prevention of cardiovascular complications in patients with AH.
The simultaneous UPLC-MS/MS determination of emerging drug combination; candesartan and chlorthalidone in human plasma and its application.[Pubmed:28178366]
Biomed Chromatogr. 2017 Sep;31(9).
A novel, precise, sensitive and accurate ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method has been developed for the simultaneous determination of a novel drug combination, Candesartan (CAN) and chlorthalidone (CHL), in human plasma. Chromatographic separation was achieved on Waters Acquity UPLC BEH C18 (50 x 2.1 mm, 1.7 mum). Mobile phase consisting of 1 mm ammonium acetate in water-acetonitrile (20:80 v/v) was used. The total chromatographic runtime was 1.9 min with retention times for CAN and CHL at 0.7 and 1.1 min respectively. Ionization and detection of analytes and internal standards was performed on a triple quadrupole mass spectrometer, operating in the multiple reaction monitoring and negative ionization mode. Quantitation was done to monitor protonated precursor --> product ion transition of m/z 439.2 --> 309.0 for CAN, 337.0 --> 189.8 for CHL and 443.2 --> 312.1 for Candesartan D4 and 341.0 --> 189.8 for chlorthalidone D4. The method was validated over a wide dynamic concentration range of 2.0-540.0 ng/mL for Candesartan and 1.0-180.0 ng/mL for chlorthalidone. The validated method was successfully applied for the assay of CAN and CHL in healthy volunteers.
The effect of candesartan on pentraxin-3 plasma levels as marker of endothelial dysfunction in patients with essential arterial hypertension.[Pubmed:28220370]
Ir J Med Sci. 2017 Aug;186(3):621-629.
BACKGROUND: In the last decades, the studies performed on the field of endothelial dysfunction confirmed the fact that the starting point of this pathology is the inflammation. Several inflammatory biomarkers had been discovered and studied, ones showing systemic inflammation, and others being more specific biomarkers and showing the local inflammation. Pentraxin-3 (PTX3) is a new inflammatory biomarker, from the same family as high-selectivity C-reactive protein (hs-CRP), but it is a more specific biomarker, due to its local production: the endothelial cells and not the liver like in the case of hs-CRP. AIMS: Several antihypertensive classes of drugs seem to have a positive impact on reducing the local endothelial inflammation, beyond their effect of lowering the blood pressure, so this study aims to analyze the effect of Candesartan on the two inflammatory biomarkers: PTX3 and CRP, compared with other antihypertensive drugs, in hypertensive patients with endothelial dysfunction. METHODS: A total of 365 patients were included in the study: 127 hypertensive patients were under treatment with Candesartan, 134 patients were under treatment with other hypotensive medication (beta blockers, calcium channel blockers, and diuretics), both groups with controlled values of blood pressure, and 104 were normotensive persons. Classical methods of assessing the endothelial function were correlated with these biochemical markers. RESULTS: The patients treated with Candesartan had a significant lower value of PTX3 and hs-CRP, compared with those under treatment with other antihypertensive medication as follows: PTX3: 0.61 +/- 0.49 vs 0.95 +/- 1.04 ng/ml, P = 0.006 and hs-CRP: 0.19 +/- 0.20 vs 0.20 +/- 0.22 mg/dl, P = 0.54. CONCLUSIONS: Candesartan decreases PTX3 and hs-CRP plasma levels more powerful than other classes of antihypertensive drugs (beta blockers, calcium channel blockers, and diuretics), so we may assume that Candesartan has a more potent action in reversing endothelial dysfunction and that it offers a higher vascular protection than other classes of antihypertensive drugs. We are suggesting that this new biochemical marker, PTX3, might be better and more specific marker for endothelial dysfunction, than hs-CRP.


