GSK J2CAS# 1394854-52-4 |
- MPI-0479605
Catalog No.:BCC5347
CAS No.:1246529-32-7
- Kif15-IN-1
Catalog No.:BCC5152
CAS No.:672926-32-8
- Kif15-IN-2
Catalog No.:BCC5153
CAS No.:672926-33-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1394854-52-4 | SDF | Download SDF |
| PubChem ID | 73010924 | Appearance | Powder |
| Formula | C22H23N5O2 | M.Wt | 389.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 42 mg/mL (107.84 mM) *"≥" means soluble, but saturation unknown. | ||
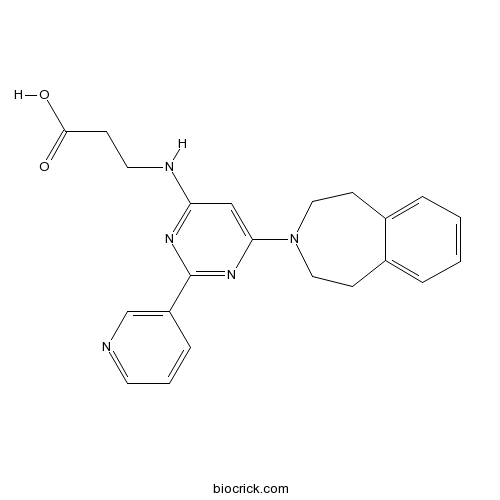
| Chemical Name | 3-[[2-pyridin-3-yl-6-(1,2,4,5-tetrahydro-3-benzazepin-3-yl)pyrimidin-4-yl]amino]propanoic acid | ||
| SMILES | C1CN(CCC2=CC=CC=C21)C3=CC(=NC(=N3)C4=CN=CC=C4)NCCC(=O)O | ||
| Standard InChIKey | LJIFOCRGDDQFJF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H23N5O2/c28-21(29)7-11-24-19-14-20(26-22(25-19)18-6-3-10-23-15-18)27-12-8-16-4-1-2-5-17(16)9-13-27/h1-6,10,14-15H,7-9,11-13H2,(H,28,29)(H,24,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inactive control of GSK J1 (Cat No.4593) (IC50 > 100 μM for inhibition of JMJD3/UTX). Cell permeable ester derivative, GSK J5, also available. |

GSK J2 Dilution Calculator

GSK J2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5677 mL | 12.8386 mL | 25.6772 mL | 51.3545 mL | 64.1931 mL |
| 5 mM | 0.5135 mL | 2.5677 mL | 5.1354 mL | 10.2709 mL | 12.8386 mL |
| 10 mM | 0.2568 mL | 1.2839 mL | 2.5677 mL | 5.1354 mL | 6.4193 mL |
| 50 mM | 0.0514 mL | 0.2568 mL | 0.5135 mL | 1.0271 mL | 1.2839 mL |
| 100 mM | 0.0257 mL | 0.1284 mL | 0.2568 mL | 0.5135 mL | 0.6419 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK-J2 is an isomer of GSK-J1 that does not have any specific activity. GSK-J1 is a potent inhibitor of H3K27me3/me2-demethylases JMJD3/KDM6B and UTX/KDM6A.
In Vitro:GSK-J2 is an isomer of GSK-J1, and shows poor activity towards KDM6A and KDM6B, with IC50 of > 100 μM and 49 μM, respectively[1].
References:
[1]. Heinemann B, et al. Inhibition of demethylases by GSK-J1/J4. Nature. 2014 Oct 2;514(7520):E1-2.
- GSK J5
Catalog No.:BCC6264
CAS No.:1394854-51-3
- Trityl candesartan
Catalog No.:BCC9187
CAS No.:139481-72-4
- Candesartan methyl ester
Catalog No.:BCC8902
CAS No.:139481-69-9
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Candesartan ethyl ester
Catalog No.:BCC8901
CAS No.:139481-58-6
- Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate
Catalog No.:BCC9032
CAS No.:139481-44-0
- Ethyl 2-ethoxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylate
Catalog No.:BCC8970
CAS No.:139481-41-7
- Methyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC9033
CAS No.:139481-28-0
- 6-O-apiosyl-5-O-Methylvisammioside
Catalog No.:BCN7858
CAS No.:139446-82-5
- Boc-Cysteinol(Bzl)
Catalog No.:BCC3043
CAS No.:139428-96-9
- 8alpha-Hydroxyhirsutinolide
Catalog No.:BCN7111
CAS No.:1394156-45-6
- GNE-317
Catalog No.:BCC5655
CAS No.:1394076-92-6
- MS436
Catalog No.:BCC4037
CAS No.:1395084-25-9
- Buddlejasaponin IV
Catalog No.:BCN5344
CAS No.:139523-30-1
- 2-(7-Methoxy-1-naphthyl)ethylamine hydrochloride
Catalog No.:BCN1574
CAS No.:139525-77-2
- Fmoc-Leu-ol
Catalog No.:BCC2582
CAS No.:139551-83-0
- Cannabidiol
Catalog No.:BCN6208
CAS No.:13956-29-1
- Lycoclavanol
Catalog No.:BCN6209
CAS No.:13956-51-9
- Serratriol
Catalog No.:BCN6210
CAS No.:13956-52-0
- Epicannabidiol hydrate
Catalog No.:BCN6207
CAS No.:139561-95-8
- 3-Bromoisonicotinic Acid
Catalog No.:BCC8368
CAS No.:13959-02-9
- Purotoxin 1
Catalog No.:BCC6333
CAS No.:1396322-38-5
- PR 39 (porcine)
Catalog No.:BCC5856
CAS No.:139637-11-9
- CGP 52432
Catalog No.:BCC6989
CAS No.:139667-74-6
A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response.[Pubmed:22842901]
Nature. 2012 Aug 16;488(7411):404-8.
The jumonji (JMJ) family of histone demethylases are Fe2+- and alpha-ketoglutarate-dependent oxygenases that are essential components of regulatory transcriptional chromatin complexes. These enzymes demethylate lysine residues in histones in a methylation-state and sequence-specific context. Considerable effort has been devoted to gaining a mechanistic understanding of the roles of histone lysine demethylases in eukaryotic transcription, genome integrity and epigenetic inheritance, as well as in development, physiology and disease. However, because of the absence of any selective inhibitors, the relevance of the demethylase activity of JMJ enzymes in regulating cellular responses remains poorly understood. Here we present a structure-guided small-molecule and chemoproteomics approach to elucidating the functional role of the H3K27me3-specific demethylase subfamily (KDM6 subfamily members JMJD3 and UTX). The liganded structures of human and mouse JMJD3 provide novel insight into the specificity determinants for cofactor, substrate and inhibitor recognition by the KDM6 subfamily of demethylases. We exploited these structural features to generate the first small-molecule catalytic site inhibitor that is selective for the H3K27me3-specific JMJ subfamily. We demonstrate that this inhibitor binds in a novel manner and reduces lipopolysaccharide-induced proinflammatory cytokine production by human primary macrophages, a process that depends on both JMJD3 and UTX. Our results resolve the ambiguity associated with the catalytic function of H3K27-specific JMJs in regulating disease-relevant inflammatory responses and provide encouragement for designing small-molecule inhibitors to allow selective pharmacological intervention across the JMJ family.


