MS436BRD4 inhibitor CAS# 1395084-25-9 |
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- GSK 525768A
Catalog No.:BCC1603
CAS No.:1260530-25-3
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- CPI-203
Catalog No.:BCC4099
CAS No.:1446144-04-2
- OTX-015
Catalog No.:BCC1829
CAS No.:202590-98-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1395084-25-9 | SDF | Download SDF |
| PubChem ID | 60171585 | Appearance | Powder |
| Formula | C18H17N5O3S | M.Wt | 383.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 25.5 mg/mL (66.51 mM; Need ultrasonic and warming) | ||
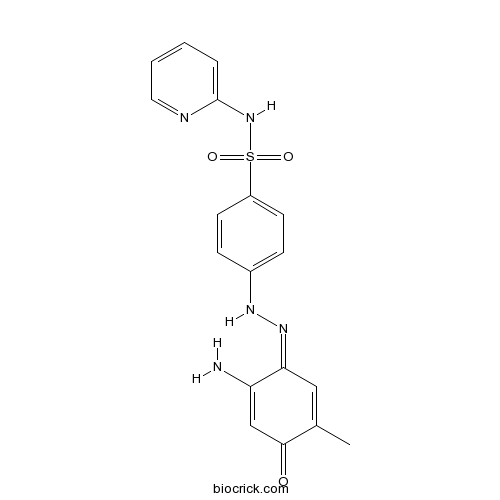
| Chemical Name | 4-[(2Z)-2-(2-amino-5-methyl-4-oxocyclohexa-2,5-dien-1-ylidene)hydrazinyl]-N-pyridin-2-ylbenzenesulfonamide | ||
| SMILES | CC1=CC(=NNC2=CC=C(C=C2)S(=O)(=O)NC3=CC=CC=N3)C(=CC1=O)N | ||
| Standard InChIKey | BBJDRANVVNBXAR-JWGURIENSA-N | ||
| Standard InChI | InChI=1S/C18H17N5O3S/c1-12-10-16(15(19)11-17(12)24)22-21-13-5-7-14(8-6-13)27(25,26)23-18-4-2-3-9-20-18/h2-11,21H,19H2,1H3,(H,20,23)/b22-16- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective BRD4 bromodomain inhibitor (Ki = 30 - 50 nM for the first bromodomain (BRD4(1)). Exhibits 10-fold selectivity for BRD4(1) over BRD4(2). Blocks BRD4 transcriptional activity in lipopolysaccharide-induced production of both nitric oxide and IL-6 in mouse macrophages (IC50 values are 3.8 and 4.9 μM, respectively). Attenuates melanoma cell proliferation in vitro. |

MS436 Dilution Calculator

MS436 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6081 mL | 13.0405 mL | 26.0811 mL | 52.1621 mL | 65.2026 mL |
| 5 mM | 0.5216 mL | 2.6081 mL | 5.2162 mL | 10.4324 mL | 13.0405 mL |
| 10 mM | 0.2608 mL | 1.3041 mL | 2.6081 mL | 5.2162 mL | 6.5203 mL |
| 50 mM | 0.0522 mL | 0.2608 mL | 0.5216 mL | 1.0432 mL | 1.3041 mL |
| 100 mM | 0.0261 mL | 0.1304 mL | 0.2608 mL | 0.5216 mL | 0.652 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
MS436 is a potent and selective small-molecule inhibitor of BRD4 with Ki values of <0.085μM and 0.34μM, respectively for BrD1 and BrD2 [1].
BRD4 plays a role in gene transcription and is a drug target for cancer and inflammation. It has two bromodomains. MS436 is a diazobenzene compound, it is designed from the SAR studies to have higher selectivity. In vitro fluorescent anisotropy assay shows MS436 has about 10-fold higher affinity of BrD1 over BrD2. MS436 binds to BRD4 through a set of water-mediated interaction and this is the molecular basis for the binding affinity. MS436 also has activity to CBP BrD. In RAW264.7 cells, MS436 can block NF-κB-directed NO production and block the expression of proinflammatory cytokine interleukin (IL)-6 induced by LPS [1].
References:
[1] Zhang G, Plotnikov AN, Rusinova E, Shen T, Morohashi K, Joshua J, Zeng L, Mujtaba S, Ohlmeyer M, Zhou MM. Structure-guided design of potent diazobenzene inhibitors for the BET bromodomains. J Med Chem. 2013 Nov 27;56(22):9251-64.
- GSK J2
Catalog No.:BCC6263
CAS No.:1394854-52-4
- GSK J5
Catalog No.:BCC6264
CAS No.:1394854-51-3
- Trityl candesartan
Catalog No.:BCC9187
CAS No.:139481-72-4
- Candesartan methyl ester
Catalog No.:BCC8902
CAS No.:139481-69-9
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Candesartan ethyl ester
Catalog No.:BCC8901
CAS No.:139481-58-6
- Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate
Catalog No.:BCC9032
CAS No.:139481-44-0
- Ethyl 2-ethoxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylate
Catalog No.:BCC8970
CAS No.:139481-41-7
- Methyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC9033
CAS No.:139481-28-0
- 6-O-apiosyl-5-O-Methylvisammioside
Catalog No.:BCN7858
CAS No.:139446-82-5
- Boc-Cysteinol(Bzl)
Catalog No.:BCC3043
CAS No.:139428-96-9
- 8alpha-Hydroxyhirsutinolide
Catalog No.:BCN7111
CAS No.:1394156-45-6
- Buddlejasaponin IV
Catalog No.:BCN5344
CAS No.:139523-30-1
- 2-(7-Methoxy-1-naphthyl)ethylamine hydrochloride
Catalog No.:BCN1574
CAS No.:139525-77-2
- Fmoc-Leu-ol
Catalog No.:BCC2582
CAS No.:139551-83-0
- Cannabidiol
Catalog No.:BCN6208
CAS No.:13956-29-1
- Lycoclavanol
Catalog No.:BCN6209
CAS No.:13956-51-9
- Serratriol
Catalog No.:BCN6210
CAS No.:13956-52-0
- Epicannabidiol hydrate
Catalog No.:BCN6207
CAS No.:139561-95-8
- 3-Bromoisonicotinic Acid
Catalog No.:BCC8368
CAS No.:13959-02-9
- Purotoxin 1
Catalog No.:BCC6333
CAS No.:1396322-38-5
- PR 39 (porcine)
Catalog No.:BCC5856
CAS No.:139637-11-9
- CGP 52432
Catalog No.:BCC6989
CAS No.:139667-74-6
- EPZ005687
Catalog No.:BCC2219
CAS No.:1396772-26-1
Structure-guided design of potent diazobenzene inhibitors for the BET bromodomains.[Pubmed:24144283]
J Med Chem. 2013 Nov 27;56(22):9251-64.
BRD4, characterized by two acetyl-lysine binding bromodomains and an extra-terminal (ET) domain, is a key chromatin organizer that directs gene activation in chromatin through transcription factor recruitment, enhancer assembly, and pause release of the RNA polymerase II complex for transcription elongation. BRD4 has been recently validated as a new epigenetic drug target for cancer and inflammation. Our current knowledge of the functional differences of the two bromodomains of BRD4, however, is limited and is hindered by the lack of selective inhibitors. Here, we report our structure-guided development of diazobenzene-based small-molecule inhibitors for the BRD4 bromodomains that have over 90% sequence identity at the acetyl-lysine binding site. Our lead compound, MS436, through a set of water-mediated interactions, exhibits low nanomolar affinity (estimated Ki of 30-50 nM), with preference for the first bromodomain over the second. We demonstrated that MS436 effectively inhibits BRD4 activity in NF-kappaB-directed production of nitric oxide and proinflammatory cytokine interleukin-6 in murine macrophages. MS436 represents a new class of bromodomain inhibitors and will facilitate further investigation of the biological functions of the two bromodomains of BRD4 in gene expression.
BRD4 sustains melanoma proliferation and represents a new target for epigenetic therapy.[Pubmed:23950209]
Cancer Res. 2013 Oct 15;73(20):6264-76.
Metastatic melanoma remains a mostly incurable disease. Although newly approved targeted therapies are efficacious in a subset of patients, resistance and relapse rapidly ensue. Alternative therapeutic strategies to manipulate epigenetic regulators and disrupt the transcriptional program that maintains tumor cell identity are emerging. Bromodomain and extraterminal domain (BET) proteins are epigenome readers known to exert key roles at the interface between chromatin remodeling and transcriptional regulation. Here, we report that BRD4, a BET family member, is significantly upregulated in primary and metastatic melanoma tissues compared with melanocytes and nevi. Treatment with BET inhibitors impaired melanoma cell proliferation in vitro and tumor growth and metastatic behavior in vivo, effects that were mostly recapitulated by individual silencing of BRD4. RNA sequencing of BET inhibitor-treated cells followed by Gene Ontology analysis showed a striking impact on transcriptional programs controlling cell growth, proliferation, cell-cycle regulation, and differentiation. In particular, we found that, rapidly after BET displacement, key cell-cycle genes (SKP2, ERK1, and c-MYC) were downregulated concomitantly with the accumulation of cyclin-dependent kinase (CDK) inhibitors (p21 and p27), followed by cell-cycle arrest. Importantly, BET inhibitor efficacy was not influenced by BRAF or NRAS mutational status, opening the possibility of using these small-molecule compounds to treat patients for whom no effective targeted therapy exists. Collectively, our study reveals a critical role for BRD4 in melanoma tumor maintenance and renders it a legitimate and novel target for epigenetic therapy directed against the core transcriptional program of melanoma.


