8alpha-HydroxyhirsutinolideCAS# 1394156-45-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1394156-45-6 | SDF | Download SDF |
| PubChem ID | 70690654 | Appearance | Powder |
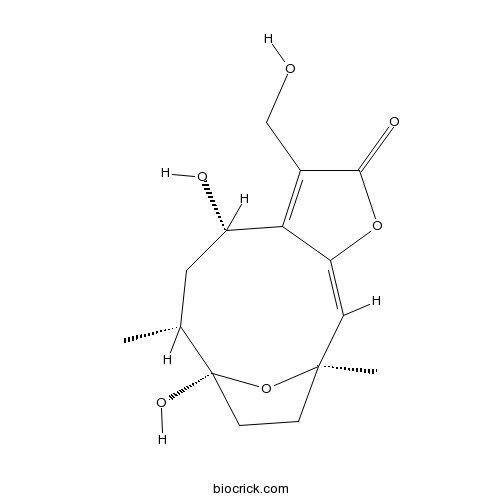
| Formula | C15H20O6 | M.Wt | 296.31 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,2E,8S,10R,11S)-8,11-dihydroxy-6-(hydroxymethyl)-1,10-dimethyl-4,14-dioxatricyclo[9.2.1.03,7]tetradeca-2,6-dien-5-one | ||
| SMILES | CC1CC(C2=C(C(=O)OC2=CC3(CCC1(O3)O)C)CO)O | ||
| Standard InChIKey | HGVUPZFNJFDVQM-HEQUYQGPSA-N | ||
| Standard InChI | InChI=1S/C15H20O6/c1-8-5-10(17)12-9(7-16)13(18)20-11(12)6-14(2)3-4-15(8,19)21-14/h6,8,10,16-17,19H,3-5,7H2,1-2H3/b11-6+/t8-,10+,14-,15+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 8alpha-Hydroxyhirsutinolide shows anti-inflammatory activity, it inhibits TNF-α-induced NF-κB activity with the IC(50) value of 1.9 uM. |
| Targets | NO | TNF-α | NF-kB |

8alpha-Hydroxyhirsutinolide Dilution Calculator

8alpha-Hydroxyhirsutinolide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3748 mL | 16.8742 mL | 33.7484 mL | 67.4969 mL | 84.3711 mL |
| 5 mM | 0.675 mL | 3.3748 mL | 6.7497 mL | 13.4994 mL | 16.8742 mL |
| 10 mM | 0.3375 mL | 1.6874 mL | 3.3748 mL | 6.7497 mL | 8.4371 mL |
| 50 mM | 0.0675 mL | 0.3375 mL | 0.675 mL | 1.3499 mL | 1.6874 mL |
| 100 mM | 0.0337 mL | 0.1687 mL | 0.3375 mL | 0.675 mL | 0.8437 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- GNE-317
Catalog No.:BCC5655
CAS No.:1394076-92-6
- Tiotropium Bromide hydrate
Catalog No.:BCC4585
CAS No.:139404-48-1
- Guan-fu base A
Catalog No.:BCN8491
CAS No.:1394-48-5
- TC LPA5 4
Catalog No.:BCC6267
CAS No.:1393814-38-4
- KPT-330
Catalog No.:BCC4446
CAS No.:1393477-72-9
- Thiostrepton
Catalog No.:BCC7621
CAS No.:1393-48-2
- KN-93
Catalog No.:BCC1683
CAS No.:139298-40-1
- MDL 100907
Catalog No.:BCC7877
CAS No.:139290-65-6
- Zolmitriptan
Catalog No.:BCC5062
CAS No.:139264-17-8
- H2L5186303
Catalog No.:BCC6315
CAS No.:139262-76-3
- Fmoc-Lys-OH.HCl
Catalog No.:BCC3512
CAS No.:139262-23-0
- G-36
Catalog No.:BCC6283
CAS No.:1392487-51-2
- Boc-Cysteinol(Bzl)
Catalog No.:BCC3043
CAS No.:139428-96-9
- 6-O-apiosyl-5-O-Methylvisammioside
Catalog No.:BCN7858
CAS No.:139446-82-5
- Methyl 2-(((2'-cyano-[1,1'-biphenyl]-4-yl)methyl)amino)-3-nitrobenzoate
Catalog No.:BCC9033
CAS No.:139481-28-0
- Ethyl 2-ethoxy-1-[(2'-cyanobiphenyl-4-yl)methyl]-1H-benzimidazole-7-carboxylate
Catalog No.:BCC8970
CAS No.:139481-41-7
- Methyl 1-[(2'-cyanobiphenyl-4-yl)methyl]-2-ethoxy-1H-benzimidazole-7-carboxylate
Catalog No.:BCC9032
CAS No.:139481-44-0
- Candesartan ethyl ester
Catalog No.:BCC8901
CAS No.:139481-58-6
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Candesartan methyl ester
Catalog No.:BCC8902
CAS No.:139481-69-9
- Trityl candesartan
Catalog No.:BCC9187
CAS No.:139481-72-4
- GSK J5
Catalog No.:BCC6264
CAS No.:1394854-51-3
- GSK J2
Catalog No.:BCC6263
CAS No.:1394854-52-4
- MS436
Catalog No.:BCC4037
CAS No.:1395084-25-9
Anti-inflammatory sesquiterpene lactones from the flower of Vernonia cinerea.[Pubmed:22850207]
Bioorg Med Chem Lett. 2012 Sep 1;22(17):5559-62.
Bioassay-guided fractionation of the hexane extract from the flowers of Vernonia cinerea (Asteraceae) led to the isolation of a new sesquiterpene lactone, 8alpha-Hydroxyhirsutinolide (2), and a new naturally occurring derivative, 8alpha-hydroxyl-1-O-methylhirsutinolide (3), along with seven known compounds (1 and 4-9). The structures of the new compounds were determined by 1D and 2D NMR experiments and by comparison with the structure of compound 1, whose relative stereochemistry was determined by X-ray analysis. The isolated compounds were evaluated for their cancer chemopreventive potential based on their ability to inhibit nitric oxide (NO) production and tumor necrosis factor alpha (TNF-alpha)-induced NF-kappaB activity. Compounds 1, 2, 4, 5, and 9 inhibited TNF-alpha-induced NF-kappaB activity with IC(50) values of 3.1, 1.9, 0.6, 5.2, and 1.6 muM, respectively; compounds 4 and 6-9 exhibited significant NO inhibitory activity with IC(50) values of 2.0, 1.5, 1.2, 2.7, and 2.4 muM, respectively.


