GSK 525768Ainactive stereoisomer of I-BET-762 CAS# 1260530-25-3 |
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- MS436
Catalog No.:BCC4037
CAS No.:1395084-25-9
- OTX-015
Catalog No.:BCC1829
CAS No.:202590-98-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1260530-25-3 | SDF | Download SDF |
| PubChem ID | 52934127 | Appearance | Powder |
| Formula | C22H22ClN5O2 | M.Wt | 423.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (235.90 mM) *"≥" means soluble, but saturation unknown. | ||
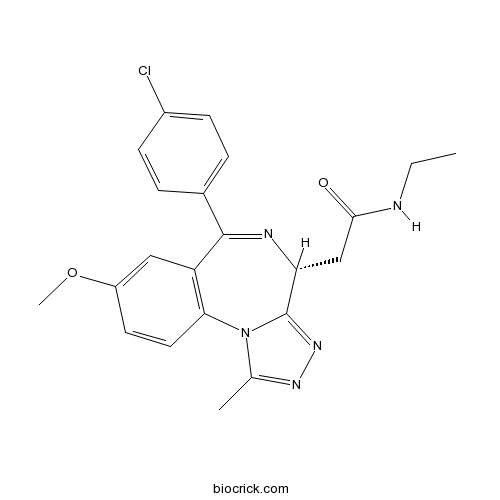
| Chemical Name | 2-[(4R)-6-(4-chlorophenyl)-8-methoxy-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepin-4-yl]-N-ethylacetamide | ||
| SMILES | CCNC(=O)CC1C2=NN=C(N2C3=C(C=C(C=C3)OC)C(=N1)C4=CC=C(C=C4)Cl)C | ||
| Standard InChIKey | AAAQFGUYHFJNHI-GOSISDBHSA-N | ||
| Standard InChI | InChI=1S/C22H22ClN5O2/c1-4-24-20(29)12-18-22-27-26-13(2)28(22)19-10-9-16(30-3)11-17(19)21(25-18)14-5-7-15(23)8-6-14/h5-11,18H,4,12H2,1-3H3,(H,24,29)/t18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

GSK 525768A Dilution Calculator

GSK 525768A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.359 mL | 11.7952 mL | 23.5905 mL | 47.1809 mL | 58.9762 mL |
| 5 mM | 0.4718 mL | 2.359 mL | 4.7181 mL | 9.4362 mL | 11.7952 mL |
| 10 mM | 0.2359 mL | 1.1795 mL | 2.359 mL | 4.7181 mL | 5.8976 mL |
| 50 mM | 0.0472 mL | 0.2359 mL | 0.4718 mL | 0.9436 mL | 1.1795 mL |
| 100 mM | 0.0236 mL | 0.118 mL | 0.2359 mL | 0.4718 mL | 0.5898 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK 525768A is the inactive stereoisomer of I-BET-762 which is a selective inhibitor of BET family with IC50 value of 35 nM [1].
BET (bromodomain and extra-terminal domain) has 4 members, BRD2, BDR3, BRD4 and BRDT and plays an important role in regulating transcription. It has been shown that BET involves in the process of suppressing proinflammatory cytokines production by macrophages and has potent anti-proliferative effects on tumor cells [2].
I-BET-762 is a potent BET inhibitor and suppresses macrophage-derived proinflammatory cytokines production [1]. When tested with naïve CD4+ T cells, I-BET-762 treatment for short 2-d up-regulated anti-inflammatory cytokines production (IL-10, Lag 3 and Egr2) and down-regulated proinflammatory cytokines production (GM-CSF and IL-17) constantly [3].
When tested with LPS-treated C57BL/6 mice, administration with I-BET-762 (5 mg per kg, i.p.) significantly suppressed LPS-induced acute inflammation compared with control GSK 525768A treated group [1]. In adoptive transfer of EAE mouse model with 2D2 T cell receptor that producing IL-17, pretreated T cells with I-BET-762 suppressed T cell mediated inflammation and decreased macrophages recruitment compared with GSK 525768A treatment [3].
References:
[1]. Nicodeme, E., et al., Suppression of inflammation by a synthetic histone mimic. Nature, 2010. 468(7327): p. 1119-23.
[2]. Bartholomeeusen, K., et al., Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J Biol Chem, 2012. 287(43): p. 36609-16.
[3]. Bandukwala, H.S., et al., Selective inhibition of CD4+ T-cell cytokine production and autoimmunity by BET protein and c-Myc inhibitors. Proc Natl Acad Sci U S A, 2012. 109(36): p. 14532-7.
- 3-O-beta-Allopyranosyl-(1->4)-beta-oleandropyranosyl-11-O-isobutyryl-12-O-acetyltenacigenin B
Catalog No.:BCN6765
CAS No.:1260252-18-3
- Birinapant (TL32711)
Catalog No.:BCC2250
CAS No.:1260251-31-7
- TCS 21311
Catalog No.:BCC2443
CAS No.:1260181-14-3
- 3-Oxo-21alpha-methoxy-24,25,26,27-tetranortirucall-7-ene-23(21)-lactone
Catalog No.:BCN7028
CAS No.:1260173-73-6
- TAK-438
Catalog No.:BCC1182
CAS No.:1260141-27-2
- 28-Deoxonimbolide
Catalog No.:BCN4717
CAS No.:126005-94-5
- Carminic acid
Catalog No.:BCN6541
CAS No.:1260-17-9
- Phlegmanol C
Catalog No.:BCN6138
CAS No.:1260-05-5
- Polygalic acid
Catalog No.:BCN3172
CAS No.:1260-04-4
- Oxethazaine
Catalog No.:BCC3832
CAS No.:126-27-2
- Sarsasapogenin
Catalog No.:BCN1269
CAS No.:126-19-2
- Solasodine
Catalog No.:BCN2346
CAS No.:126-17-0
- Atractyloside A
Catalog No.:BCN5383
CAS No.:126054-77-1
- 6-O-Methylcerevisterol
Catalog No.:BCN6139
CAS No.:126060-09-1
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Rubiyunnanin C
Catalog No.:BCN8045
CAS No.:1261030-04-9
- 11-Oxo-mogroside V
Catalog No.:BCN2509
CAS No.:126105-11-1
- Siamenoside I
Catalog No.:BCN2540
CAS No.:126105-12-2
- GNF179
Catalog No.:BCC5175
CAS No.:1261114-01-5
- BAPTA-AM
Catalog No.:BCC5456
CAS No.:126150-97-8
- Icariside E5
Catalog No.:BCN6140
CAS No.:126176-79-2
- Spiranthol A
Catalog No.:BCN7893
CAS No.:126192-35-6
- LY2886721
Catalog No.:BCC3807
CAS No.:1262036-50-9
- Ginsenoside Rg4
Catalog No.:BCN2713
CAS No.:126223-28-7
Remote limb ischemic post conditioning during early reperfusion alleviates cerebral ischemic reperfusion injury via GSK-3beta/CREB/ BDNF pathway.[Pubmed:28341347]
Eur J Pharmacol. 2017 May 15;803:84-93.
Remote limb ischemic post conditioning (RIPOC) has been reported to attenuate cerebral ischemic reperfusion (I/R) injury, while the molecular mechanisms remain elusive. Various studies have highlighted the involvement of glycogen synthase kinase (GSK-3beta) in cerebral I/R injury and cognitive disorders. Hence, the present study was designed to explore the role of GSK-3beta and its downstream regulators in RIPOC mediated neuroprotection against cerebral I/R injury and associated cognitive impairment. Male Wistar rats are randomly assigned into four groups: Sham, bilateral common carotid arteries occlusion (BCCAO), RIPOC and BCCAO+RIPOC. BCCAO was achieved by transient occlusion of bilateral common carotid arteries for 20min, followed by reperfusion. Non-invasive RIPOC was induced by 3 cycles each of 10min occlusion and reperfusion of both femoral arteries by using tourniquets, during early reperfusion phase. A battery of behavioral and cognitive tests were performed. Biochemical estimation of oxidative markers, anti-oxidants and pro-inflammatory markers were estimated. Levels of GSK-3beta, CREB and BDNF were estimated to confirm the molecular mechanism. Hippocampal structural abnormalities were confirmed by H and E staining. The neurobehavioral analysis revealed that neurological and cognitive deficits caused by BCCAO, were reduced by RIPOC intervention. Meanwhile, the results of biochemical tests suggested that RIPOC attenuates the BCCAO induced oxidative damage, neuroinflammation and hippocampal structural abnormalities. Further, RIPOC prevented the elevation of BCCAO induced GSK-3beta. RIPOC exerts neuroprotective effect against I/R injury, putatively by attenuating GSK-3beta expression and upregulating the levels of CREB and BDNF.
SLM, a novel carbazole-based fluorophore attenuates okadaic acid-induced tau hyperphosphorylation via down-regulating GSK-3beta activity in SH-SY5Y cells.[Pubmed:28359686]
Eur J Pharm Sci. 2017 Dec 15;110:101-108.
Phosphorylated tau dissociates from microtubules and aggregates to form neurofibrillary tangles resulting in neuronal toxicity and cognitive deficits. Attenuating tau hyperphosphorylation is considered as an effective therapeutic approach for Alzheimer's disease (AD). From our previous study, SLM, a carbazole-based fluorophore prevents Abeta aggregation, reduced glycogen synthase kinase-3beta (GSK-3beta) activity and tau hyperphosphorylation in triple transgenic mouse model of AD. However, the mechanism by which SLM attenuates tau hyperphosphorylation warrants further investigation. In the current study, we intend to evaluate the effects of SLM against okadaic acid (OA)-induced tau hyperphosphorylation and microtubules instability in human neuroblastoma (SH-SY5Y) cells. The results showed that, SLM reduced the OA-induced cell neurotoxicity and tau hyperphosphorylation in SH-SY5Y cells. SLM treatment down-regulated GSK-3beta activity. However, in the presence of GSK-3beta inhibitor (SB216763, 10muM), SLM treatment could not reduce GSK-3beta activity and tau hyperphosphorylation as compared with SB216763 treatment alone. Furthermore, SLM treatment also ameliorated OA-induced microtubules instability and cytoskeleton damage. Collectively, SLM attenuated OA-induced tau hyperphosphorylation via down-regulating GSK-3beta activity in SH-SY5Y cells. Therefore, this study supports SLM as a potential compound for AD and other tau pathology-related neurodegenerative disorders.
Transient Cerebral Ischemia Alters GSK-3beta and p-GSK-3beta Immunoreactivity in Pyramidal Neurons and Induces p-GSK-3beta Expression in Astrocytes in the Gerbil Hippocampal CA1 Area.[Pubmed:28349361]
Neurochem Res. 2017 Aug;42(8):2305-2313.
Glycogen synthase kinase 3beta (GSK-3beta) is a key downstream protein in the PI3K/Akt pathway. Phosphorylation of serine 9 of GSK-3beta (GSK-3beta activity inhibition) promotes cell survival. In this study, we examined changes in expressions of GSK-3beta and phosphorylation of GSK-3beta (p-GSK-3beta) in the gerbil hippocampal CA1 area after 5 min of transient cerebral ischemia. GSK-3beta immunoreactivity in the CA1 area was increased in pyramidal cells at 6 h after ischemia-reperfusion. It was decreased in CA1 pyramidal cells from 12 h after ischemia-reperfusion, and hardly detected in the CA1 pyramidal cells at 5 days after ischemia-reperfusion. p-GSK-3beta immunoreactivity was slightly decreased in CA1 pyramidal cells at 6 and 12 h after ischemia-reperfusion. It was significantly increased in these cells at 1 and 2 days after ischemia-reperfusion. Five days after ischemia-reperfusion, p-GSK-3beta immunoreactivity was hardly found in CA1 pyramidal cells. However, p-GSK-3beta immunoreactivity was strongly expressed in astrocytes primarily distributed in strata oriens and radiatum. In conclusion, GSK-3beta and p-GSK-3beta were significantly changed in pyramidal cells and/or astrocytes in the gerbil hippocampal CA1 area following 5 min of transient cerebral ischemia. This finding indicates that GSK-3beta and p-GSK-3beta are closely related to delayed neuronal death.
GSK-3beta Overexpression Alters the Dendritic Spines of Developmentally Generated Granule Neurons in the Mouse Hippocampal Dentate Gyrus.[Pubmed:28344548]
Front Neuroanat. 2017 Mar 10;11:18.
The dentate gyrus (DG) plays a crucial role in hippocampal-related memory. The most abundant cellular type in the DG, namely granule neurons, are developmentally generated around postnatal day P6 in mice. Moreover, a unique feature of the DG is the occurrence of adult hippocampal neurogenesis, a process that gives rise to newborn granule neurons throughout life. Adult-born and developmentally generated granule neurons share some maturational aspects but differ in others, such as in their positioning within the granule cell layer. Adult hippocampal neurogenesis encompasses a series of plastic changes that modify the function of the hippocampal trisynaptic network. In this regard, it is known that glycogen synthase kinase 3beta (GSK-3beta) regulates both synaptic plasticity and memory. By using a transgenic mouse overexpressing GSK-3beta in hippocampal neurons, we previously demonstrated that the overexpression of this kinase has deleterious effects on the maturation of newborn granule neurons. In the present study, we addressed the effects of GSK-3beta overexpression on the morphology and number of dendritic spines of developmentally generated granule neurons. To this end, we performed intracellular injections of Lucifer Yellow in developmentally generated granule neurons of wild-type and GSK-3beta-overexpressing mice and analyzed the number and morphologies of dendritic spines (namely, stubby, thin and mushroom). GSK-3beta overexpression led to a general reduction in the number of dendritic spines. In addition, it caused a slight reduction in the percentage, head diameter and length of thin spines, whereas the head diameter of mushroom spines was increased.


