OxethazaineCAS# 126-27-2 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126-27-2 | SDF | Download SDF |
| PubChem ID | 4621 | Appearance | Powder |
| Formula | C28H41N3O3 | M.Wt | 467.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Oxetacaine | ||
| Solubility | >23.2mg/mL in DMSO | ||
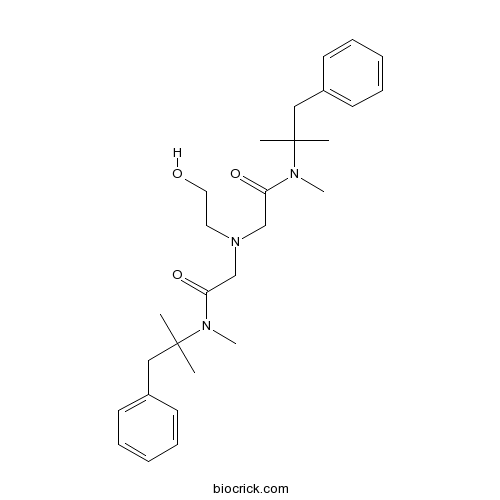
| Chemical Name | 2-[2-hydroxyethyl-[2-[methyl-(2-methyl-1-phenylpropan-2-yl)amino]-2-oxoethyl]amino]-N-methyl-N-(2-methyl-1-phenylpropan-2-yl)acetamide | ||
| SMILES | CC(C)(CC1=CC=CC=C1)N(C)C(=O)CN(CCO)CC(=O)N(C)C(C)(C)CC2=CC=CC=C2 | ||
| Standard InChIKey | FTLDJPRFCGDUFH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H41N3O3/c1-27(2,19-23-13-9-7-10-14-23)29(5)25(33)21-31(17-18-32)22-26(34)30(6)28(3,4)20-24-15-11-8-12-16-24/h7-16,32H,17-22H2,1-6H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Oxethazaine is a topical anesthetic, in preventing acid-induced esophageal pain. |

Oxethazaine Dilution Calculator

Oxethazaine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1384 mL | 10.692 mL | 21.384 mL | 42.7679 mL | 53.4599 mL |
| 5 mM | 0.4277 mL | 2.1384 mL | 4.2768 mL | 8.5536 mL | 10.692 mL |
| 10 mM | 0.2138 mL | 1.0692 mL | 2.1384 mL | 4.2768 mL | 5.346 mL |
| 50 mM | 0.0428 mL | 0.2138 mL | 0.4277 mL | 0.8554 mL | 1.0692 mL |
| 100 mM | 0.0214 mL | 0.1069 mL | 0.2138 mL | 0.4277 mL | 0.5346 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Has been used in combination with antacids for ulcer pain
- Sarsasapogenin
Catalog No.:BCN1269
CAS No.:126-19-2
- Solasodine
Catalog No.:BCN2346
CAS No.:126-17-0
- Griseofulvin
Catalog No.:BCC5327
CAS No.:126-07-8
- 10-O-Ethylcannabitriol
Catalog No.:BCN7312
CAS No.:1259515-25-7
- SCH 23390 hydrochloride
Catalog No.:BCC6849
CAS No.:125941-87-9
- VU 0155041 sodium salt
Catalog No.:BCC7642
CAS No.:1259372-69-4
- JP 1302 dihydrochloride
Catalog No.:BCC7449
CAS No.:1259314-65-2
- SAMS Peptide
Catalog No.:BCC5745
CAS No.:125911-68-4
- 24-Methylenecycloartanol acetate
Catalog No.:BCN6137
CAS No.:1259-94-5
- (+)-Glaucarubinone
Catalog No.:BCN7956
CAS No.:1259-86-5
- LY2940680
Catalog No.:BCC3935
CAS No.:1258861-20-9
- 9-O-Ethyldeacetylorientalide
Catalog No.:BCN7311
CAS No.:1258517-60-0
- Polygalic acid
Catalog No.:BCN3172
CAS No.:1260-04-4
- Phlegmanol C
Catalog No.:BCN6138
CAS No.:1260-05-5
- Carminic acid
Catalog No.:BCN6541
CAS No.:1260-17-9
- 28-Deoxonimbolide
Catalog No.:BCN4717
CAS No.:126005-94-5
- TAK-438
Catalog No.:BCC1182
CAS No.:1260141-27-2
- 3-Oxo-21alpha-methoxy-24,25,26,27-tetranortirucall-7-ene-23(21)-lactone
Catalog No.:BCN7028
CAS No.:1260173-73-6
- TCS 21311
Catalog No.:BCC2443
CAS No.:1260181-14-3
- Birinapant (TL32711)
Catalog No.:BCC2250
CAS No.:1260251-31-7
- 3-O-beta-Allopyranosyl-(1->4)-beta-oleandropyranosyl-11-O-isobutyryl-12-O-acetyltenacigenin B
Catalog No.:BCN6765
CAS No.:1260252-18-3
- GSK 525768A
Catalog No.:BCC1603
CAS No.:1260530-25-3
- Atractyloside A
Catalog No.:BCN5383
CAS No.:126054-77-1
- 6-O-Methylcerevisterol
Catalog No.:BCN6139
CAS No.:126060-09-1
Oxethazaine inhibits hepatitis B virus capsid assembly by blocking the cytosolic calcium-signalling pathway.[Pubmed:26838678]
J Gen Virol. 2016 May;97(5):1198-209.
Chronic hepatitis B virus (HBV) infection is a serious public health problem and may progress to liver fibrosis, cirrhosis and hepatocellular carcinoma. It is currently treated with PEGylated IFN-alpha2a and nucleoside/nucleotide analogues (NAs). However, PEGylated IFN treatment has problems of high cost, low efficiency and side effects. Long-term administration of NAs is necessary to avoid virus relapse, which can cause drug resistance and side effects. New efforts are now being directed to develop novel anti-HBV drugs targeting either additional viral targets other than viral DNA polymerase or host targets to improve the treatment of chronic hepatitis B. In this study, we discovered that Oxethazaine, approved for clinic use in a few countries such as Japan, India, South Africa and Brazil, can dose-dependently reduce the levels of HBV envelope antigen, extracellular HBV DNA in supernatants and intracellular HBV total DNA. However, the levels of HBV cccDNA and HBV RNAs were not affected by Oxethazaine treatment. Further study confirmed that Oxethazaine acts on the virus assembly stage of the HBV life cycle. A study of the mechanisms of Oxethazaine suggested that this drug inhibits HBV replication and capsid assembly by blocking the cytosolic calcium-signalling pathway. Moreover, Oxethazaine could inhibit the replication of lamivudine/entecavir-dual-resistant and adefovir-resistant HBV mutants. In conclusion, our study suggests that Oxethazaine may serve as a promising drug, or could be used as a starting point for anti-HBV drug discovery.
Complexation of oxethazaine with 2-hydroxypropyl-beta-cyclodextrin: increased drug solubility, decreased cytotoxicity and analgesia at inflamed tissues.[Pubmed:28211640]
J Pharm Pharmacol. 2017 Jun;69(6):652-662.
OBJECTIVES: Oxethazaine (OXZ) is one of the few local anaesthetics that provides analgesia at low pH, but presents poor solubility, cytotoxicity and no parenteral formulations. To address these issues, we aimed to prepare OXZ host-guest inclusion complex with hydroxypropyl-beta-cyclodextrin (HP-beta-CD). METHODS: The inclusion complex was formed by co-solubilization, followed by a job plot analysis to determine stoichiometry of complexation and dialysis equilibrium analysis (based on UV/VIS absorption and fluorescence profiles of OXZ). Complex formation was confirmed by phase-solubility data, X-ray, Scanning Electron Microscopy and DOSY-(1) H-NMR experiments. In vitro cytotoxicity was analysed by MTT test in 3T3 fibroblasts. In vivo analgesia was tested by Von Frey test (inflammatory wounds - rats). KEY FINDINGS: Oxethazaine complexed (1 : 1 molar ratio) with HP-beta-CD, as indicated by loss of OZX crystalline structure (X-ray) and strong host: guest interaction (NMR, K = 198/M), besides increased solubility. In vitro cell survival improved with the complex (IC50 OXZ = 28.9 mum, OXZ : HP-beta-CD = 57.8 mum). In addition, the complex (0.1% OXZ) promoted in vivo analgesia for the same time that 2% lidocaine/epinephrine did. CONCLUSION: Our results show that complexation improved physicochemical and biological properties of OXZ, allowing its application to inflamed tissues by parenteral routes.
Comparison of seven Oxethazaine containing antacids available in the Indian market.[Pubmed:24640206]
J Assoc Physicians India. 2013 Jun;61(6):400-3.
OBJECTIVE: This in-vitro study was designed to measure the quantity of acid neutralized by a suspension of a commercial antacid available in Indian markets at a labeled dose and to test the concept of the relative effectiveness of 7 different commercial antacids containing Oxethazaine. METHODS: A simple back titration methodology was used to determine the acid neutralization capacity (ANC) of antacids. RESULTS: It was observed that different antacids vary widely in their in vitro ANC. There was also a batch to batch variation noted for each brand of antacid. The analysis indicated that there was a significant difference of ANC in favor of AD versus other antacids studied. CONCLUSION: Comparison of relative effectiveness indicates that AD has highest ANC in vitro amongst other antacids. However, the present study being in vitro, the effects of antacid may vary in vivo, as individual variations also contribute to the ultimate effectiveness of an antacid.
The abuse potential of oxethazaine: effects of oxethazaine on drug-seeking behavior and analysis of its metabolites in plasma and hair in animal models.[Pubmed:23402942]
Pharmacol Biochem Behav. 2013 Apr;105:98-104.
Oxethazaine, an over-the-counter (OTC) antacid, is a precursor of phentermine, which is the most abused anorectic by methamphetamine users in Korea. However, no studies have investigated the abuse potential of Oxethazaine. Therefore, we examined and compared the consequences of Oxethazaine and phentermine treatment on animal models of conditioned place preference (CPP) and self-administration. Furthermore, Oxethazaine and its metabolites in rat plasma were monitored using liquid chromatography-tandem mass spectrometry (LC-MS/MS) after Oxethazaine administration, and compared with phentermine itself after phentermine administration to clarify the relationship between phentermine production by Oxethazaine ingestion and the possible Oxethazaine dependence. Oxethazaine metabolites were also determined by LC-MS/MS in rat hair after Oxethazaine administration to investigate the possibility of phentermine detection in hair from Oxethazaine abusers. In the behavioral experiment, phentermine (3mg/kg) produced CPP in mice while Oxethazaine (5, 10, and 15mg/kg) did not. Moreover, phentermine (0.25mg/kg/infusion) was self-administered by rats at 80% of free-feeding weight, whereas Oxethazaine was not. In the analytical study, mephentermine and phentermine, both Oxethazaine metabolites, were detected below the limit of quantitation or not detected in both plasma and hair from rats that had ingested Oxethazaine (10mg/kg, single dose or for 2weeks). On the other hand, phentermine was detected in plasma and hair samples from rats that had ingested phentermine (10mg/kg, single dose or for 2weeks). Consequently, phentermine induced significant rewarding effects, but Oxethazaine did not. Presumably, either Oxethazaine does not have any abuse potential or Oxethazaine metabolism to phentermine does not result in a pharmacologically active level of psychostimulant in the body. Furthermore, phentermine was not a major metabolite in hair obtained from Oxethazaine abusers, which should make it possible to differentiate between chronic Oxethazaine and phentermine users.


