GriseofulvinCAS# 126-07-8 |
- Vilazodone
Catalog No.:BCC2040
CAS No.:163521-12-8
- SB 271046 hydrochloride
Catalog No.:BCC1924
CAS No.:209481-24-3
- Adoprazine
Catalog No.:BCC1329
CAS No.:222551-17-9
- SEA0400
Catalog No.:BCC1941
CAS No.:223104-29-8
- Tianeptine
Catalog No.:BCC1999
CAS No.:66981-73-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126-07-8 | SDF | Download SDF |
| PubChem ID | 6713927 | Appearance | Powder |
| Formula | C17H17ClO6 | M.Wt | 352.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 33.33 mg/mL (94.48 mM; Need ultrasonic) | ||
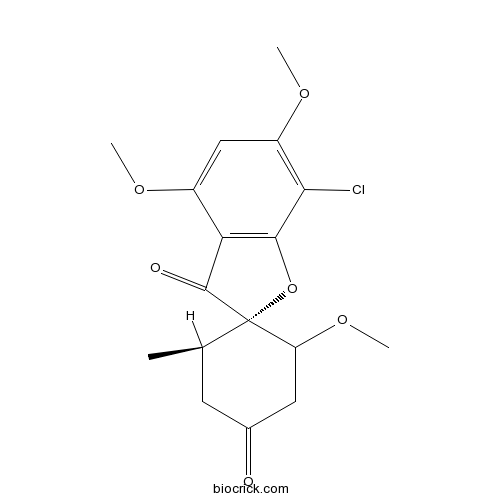
| Chemical Name | (2S,5'R)-7-chloro-3',4,6-trimethoxy-5'-methylspiro[1-benzofuran-2,4'-cyclohexane]-1',3-dione | ||
| SMILES | CC1CC(=O)CC(C12C(=O)C3=C(O2)C(=C(C=C3OC)OC)Cl)OC | ||
| Standard InChIKey | IIUZTXTZRGLYTI-LKUJNTHKSA-N | ||
| Standard InChI | InChI=1S/C17H19ClO6/c1-8-5-9(19)6-12(23-4)17(8)16(20)13-10(21-2)7-11(22-3)14(18)15(13)24-17/h7-8,12H,5-6H2,1-4H3/t8-,12?,17+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Griseofulvin Dilution Calculator

Griseofulvin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8347 mL | 14.1735 mL | 28.3471 mL | 56.6942 mL | 70.8677 mL |
| 5 mM | 0.5669 mL | 2.8347 mL | 5.6694 mL | 11.3388 mL | 14.1735 mL |
| 10 mM | 0.2835 mL | 1.4174 mL | 2.8347 mL | 5.6694 mL | 7.0868 mL |
| 50 mM | 0.0567 mL | 0.2835 mL | 0.5669 mL | 1.1339 mL | 1.4174 mL |
| 100 mM | 0.0283 mL | 0.1417 mL | 0.2835 mL | 0.5669 mL | 0.7087 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 10-O-Ethylcannabitriol
Catalog No.:BCN7312
CAS No.:1259515-25-7
- SCH 23390 hydrochloride
Catalog No.:BCC6849
CAS No.:125941-87-9
- VU 0155041 sodium salt
Catalog No.:BCC7642
CAS No.:1259372-69-4
- JP 1302 dihydrochloride
Catalog No.:BCC7449
CAS No.:1259314-65-2
- SAMS Peptide
Catalog No.:BCC5745
CAS No.:125911-68-4
- 24-Methylenecycloartanol acetate
Catalog No.:BCN6137
CAS No.:1259-94-5
- (+)-Glaucarubinone
Catalog No.:BCN7956
CAS No.:1259-86-5
- LY2940680
Catalog No.:BCC3935
CAS No.:1258861-20-9
- 9-O-Ethyldeacetylorientalide
Catalog No.:BCN7311
CAS No.:1258517-60-0
- Deacetylorientalide
Catalog No.:BCN7310
CAS No.:1258517-59-7
- Parathyroid Hormone (1-34), bovine
Catalog No.:BCC1040
CAS No.:12583-68-5
- Pristimerin
Catalog No.:BCN2315
CAS No.:1258-84-0
- Solasodine
Catalog No.:BCN2346
CAS No.:126-17-0
- Sarsasapogenin
Catalog No.:BCN1269
CAS No.:126-19-2
- Oxethazaine
Catalog No.:BCC3832
CAS No.:126-27-2
- Polygalic acid
Catalog No.:BCN3172
CAS No.:1260-04-4
- Phlegmanol C
Catalog No.:BCN6138
CAS No.:1260-05-5
- Carminic acid
Catalog No.:BCN6541
CAS No.:1260-17-9
- 28-Deoxonimbolide
Catalog No.:BCN4717
CAS No.:126005-94-5
- TAK-438
Catalog No.:BCC1182
CAS No.:1260141-27-2
- 3-Oxo-21alpha-methoxy-24,25,26,27-tetranortirucall-7-ene-23(21)-lactone
Catalog No.:BCN7028
CAS No.:1260173-73-6
- TCS 21311
Catalog No.:BCC2443
CAS No.:1260181-14-3
- Birinapant (TL32711)
Catalog No.:BCC2250
CAS No.:1260251-31-7
- 3-O-beta-Allopyranosyl-(1->4)-beta-oleandropyranosyl-11-O-isobutyryl-12-O-acetyltenacigenin B
Catalog No.:BCN6765
CAS No.:1260252-18-3
Comparative Hepatotoxicity of Fluconazole, Ketoconazole, Itraconazole, Terbinafine, and Griseofulvin in Rats.[Pubmed:28261269]
J Toxicol. 2017;2017:6746989.
Oral ketoconazole was recently the subject of regulatory safety warnings because of its association with increased risk of inducing hepatic injury. However, the relative hepatotoxicity of antifungal agents has not been clearly established. The aim of this study was to compare the hepatotoxicity induced by five commonly prescribed oral antifungal agents. Rats were treated with therapeutic oral doses of Griseofulvin, fluconazole, itraconazole, ketoconazole, and terbinafine. After 14 days, only ketoconazole had significantly higher ALT levels (p = 0.0017) and AST levels (p = 0.0008) than the control group. After 28 days, ALT levels were highest in the rats treated with ketoconazole followed by itraconazole, fluconazole, Griseofulvin, and terbinafine, respectively. The AST levels were highest in the rats treated with ketoconazole followed by itraconazole, fluconazole, terbinafine, and Griseofulvin, respectively. All drugs significantly elevated ALP levels after 14 days and 28 days of treatment (p < 0.0001). The liver enzyme levels suggested that ketoconazole had the highest risk in causing liver injury followed by itraconazole, fluconazole, terbinafine, and Griseofulvin. However, histopathological changes revealed that fluconazole was the most hepatotoxic, followed by ketoconazole, itraconazole, terbinafine, and Griseofulvin, respectively. Given the poor correlation between liver enzymes and the extent of liver injury, it is important to confirm liver injury through histological examination.
Synthesis and formulation studies of griseofulvin analogues with improved solubility and metabolic stability.[Pubmed:28258034]
Eur J Med Chem. 2017 Apr 21;130:240-247.
Griseofulvin (1) is an important antifungal agent that has recently received attention due to its antiproliferative activity in mammalian cancer cells. Comprehensive SAR studies have led to the identification of 2'-benzyloxy Griseofulvin 2, a more potent analogue with low micromolar anticancer potency in vitro. Analogue 2 was also shown to retard tumor growth through inhibition of centrosomal clustering in murine xenograft models of colon cancer and multiple myeloma. However, similar to Griseofulvin, compound 2 exhibited poor metabolic stability and aqueous solubility. In order to improve the poor pharmacokinetic properties, 11 Griseofulvin analogues were synthesized and evaluated for biological activity and physiological stabilities including SGF, plasma, and metabolic stability. Finally, the most promising compounds were investigated in respect to thermodynamic solubility and formulation studies. The 2'-benzylamine analogue 10 proved to be the most promising compound with low muM in vitro anticancer potency, a 200-fold increase in PBS solubility over compound 2, and with improved metabolic stability. Furthermore, this analogue proved compatible with formulations suitable for both oral and intravenous administration. Finally, 2'-benzylamine analogue 10 was confirmed to induce G2/M cell cycle arrest in vitro.
Griseofulvin Derivative and Indole Alkaloids from Penicillium griseofulvum CPCC 400528.[Pubmed:28117586]
J Nat Prod. 2017 Feb 24;80(2):371-376.
A new Griseofulvin derivative, 4'-demethoxy-4'-N-isopentylisoGriseofulvin (1), three new indole alkaloids, 2-demethylcyclopiamide E (2), 2-demethylsperadine F (3), and clopiamine C (4), and five known metabolites (5-9) were isolated from Penicillium griseofulvum CPCC 400528. Compound 1 is the first reported Griseofulvin analogue with an N-isopentane group and the first example of a naturally occurring N-containing Griseofulvin analogue. Their structures and absolute configurations were elucidated through extensive spectroscopic analyses, calculated ECD, and single-crystal X-ray diffraction (Cu Kalpha). The possible biogenetic pathway of 1-3 was proposed. Compounds 1, 2, and 5 exhibited anti-HIV activities with IC50 values of 33.2, 20.5, and 12.6 muM, respectively.
Griseofulvin impairs intraerythrocytic growth of Plasmodium falciparum through ferrochelatase inhibition but lacks activity in an experimental human infection study.[Pubmed:28176804]
Sci Rep. 2017 Feb 8;7:41975.
Griseofulvin, an orally active antifungal drug used to treat dermatophyte infections, has a secondary effect of inducing cytochrome P450-mediated production of N-methyl protoporphyrin IX (N-MPP). N-MPP is a potent competitive inhibitor of the heme biosynthetic-enzyme ferrochelatase, and inhibits the growth of cultured erythrocyte stage Plasmodium falciparum. Novel drugs against Plasmodium are needed to achieve malaria elimination. Thus, we investigated whether Griseofulvin shows anti-plasmodial activity. We observed that the intraerythrocytic growth of P. falciparum is inhibited in red blood cells pretreated with Griseofulvin in vitro. Treatment with 100 muM Griseofulvin was sufficient to prevent parasite growth and induce the production of N-MPP. Inclusion of the ferrochelatase substrate PPIX blocked the inhibitory activity of Griseofulvin, suggesting that Griseofulvin exerts its activity through the N-MPP-dependent inhibition of ferrochelatase. In an ex-vivo study, red blood cells from Griseofulvin-treated subjects were refractory to the growth of cultured P. falciparum. However, in a clinical trial Griseofulvin failed to show either therapeutic or prophylactic effect in subjects infected with blood stage P. falciparum. Although the development of Griseofulvin as an antimalarial is not warranted, it represents a novel inhibitor of P. falciparum growth and acts via the N-MPP-dependent inhibition of ferrochelatase.


