Parathyroid Hormone (1-34), bovineEnhancer of blood calcium level CAS# 12583-68-5 |
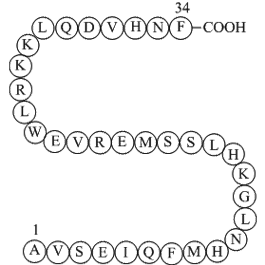
- Parathyroid hormone (1-34) (human)
Catalog No.:BCC1046
CAS No.:52232-67-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 12583-68-5 | SDF | Download SDF |
| PubChem ID | 16132279 | Appearance | Powder |
| Formula | C183H288N54O50S2 | M.Wt | 4108.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Sequence | H2N-Ala-Val-Ser-Glu-Ile-Gln-Phe-Met-His-Asn-Leu-Gly-Lys-His-Leu-Ser-Ser-Met-Glu-Arg-Val-Glu-Trp-Leu-Arg-Lys-Lys-Leu-Gln-Asp-Val-His-Asn-Phe-OH | ||
| SMILES | CCC(C)C(C(=O)NC(CCC(=O)N)C(=O)NCC(=O)NC(CCSC)C(=O)NC(CC1=CN=CN1)C(=O)NC(CC(=O)N)C(=O)NC(CC(C)C)C(=O)NCC(=O)NC(CCCCN)C(=O)NC(CC2=CN=CN2)C(=O)NC(CC(C)C)C(=O)NCC(=O)NC(CO)C(=O)NC(CCSC)C(=O)NC(CCC(=O)O)C(=O)NC(CCCNC(=N)N)C(=O)NC(C(C)C)C(=O)NC(CCC(=O)O)C(=O)NC(CC3=CNC4=CC=CC=C43)C(=O)NC(CC(C)C)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)NC(CC(C)C)C(=O)NC(CCC(=O)N)C(=O)NC(CC(=O)O)C(=O)NC(C(C)C)C(=O)NC(CC5=CN=CN5)C(=O)NC(CC(=O)N)C(=O)NC(CC6=CC=CC=C6)C(=O)O)NC(=O)C(CCC(=O)O)NC(=O)C(CO)NC(=O)C(C(C)C)NC(=O)CN | ||
| Standard InChIKey | BHCZZGBILISWFT-KANWXXSKSA-N | ||
| Standard InChI | InChI=1S/C174H278N54O49S2/c1-19-93(16)142(228-156(260)110(46-51-137(243)244)208-167(271)126(82-230)224-168(272)139(90(10)11)225-131(235)73-178)171(275)210-106(42-47-127(179)231)143(247)193-78-133(237)200-111(52-59-278-17)153(257)218-119(68-97-76-188-84-197-97)161(265)219-121(70-129(181)233)163(267)213-114(62-87(4)5)145(249)194-79-132(236)199-101(37-25-28-54-175)146(250)217-118(67-96-75-187-83-196-96)160(264)212-113(61-86(2)3)144(248)195-80-134(238)201-125(81-229)166(270)209-112(53-60-279-18)154(258)206-108(44-49-135(239)240)150(254)204-105(41-32-58-191-174(185)186)155(259)226-140(91(12)13)169(273)211-109(45-50-136(241)242)152(256)216-117(66-95-74-192-100-36-24-23-35-99(95)100)159(263)215-116(64-89(8)9)157(261)205-104(40-31-57-190-173(183)184)148(252)202-102(38-26-29-55-176)147(251)203-103(39-27-30-56-177)149(253)214-115(63-88(6)7)158(262)207-107(43-48-128(180)232)151(255)221-123(72-138(245)246)165(269)227-141(92(14)15)170(274)222-120(69-98-77-189-85-198-98)162(266)220-122(71-130(182)234)164(268)223-124(172(276)277)65-94-33-21-20-22-34-94/h20-24,33-36,74-77,83-93,101-126,139-142,192,229-230H,19,25-32,37-73,78-82,175-178H2,1-18H3,(H2,179,231)(H2,180,232)(H2,181,233)(H2,182,234)(H,187,196)(H,188,197)(H,189,198)(H,193,247)(H,194,249)(H,195,248)(H,199,236)(H,200,237)(H,201,238)(H,202,252)(H,203,251)(H,204,254)(H,205,261)(H,206,258)(H,207,262)(H,208,271)(H,209,270)(H,210,275)(H,211,273)(H,212,264)(H,213,267)(H,214,253)(H,215,263)(H,216,256)(H,217,250)(H,218,257)(H,219,265)(H,220,266)(H,221,255)(H,222,274)(H,223,268)(H,224,272)(H,225,235)(H,226,259)(H,227,269)(H,228,260)(H,239,240)(H,241,242)(H,243,244)(H,245,246)(H,276,277)(H4,183,184,190)(H4,185,186,191)/t93-,101-,102-,103-,104-,105-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,139-,140-,141-,142-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Parathyroid hormone (PTH) receptor agonist. Increases calcium and inorganic phosphate levels in the serum of young rats. Increases osteocalcin concentrations in the serum of old rats without affecting calcium or phosphate levels. |

Parathyroid Hormone (1-34), bovine Dilution Calculator

Parathyroid Hormone (1-34), bovine Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Parathyroid hormone (PTH) is the most important endocrine regulator of calcium and phosphorus concentration in extracellular fluid, which is secreted by the chief cell of the parathyroid glands as a polypeptide containing 84 amino acids. It acts to increase the concentration of calcium (Ca2+) in the blood, whereas calcitonin (a hormone produced by the parafollicular cells (C cells) of the thyroid gland) acts to decrease calcium concentration.
PTH acts to increase the concentration of calcium in the blood by acting upon the parathyroid hormone 1 receptor (high levels in bone and kidney) and the parathyroid hormone 2 receptor (high levels in the central nervous system, pancreas, testis, and placenta).
PTH half-life is approximately 4 minutes. [1] Like most other protein hormones, parathyroid hormone is synthesized as a preprohormone. After intracellular processing, the mature hormone is packaged within the Golgi into secretory vesicles, the secreted into blood by exocytosis.
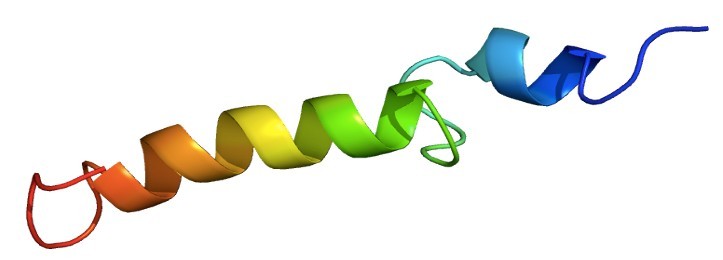
Fig 1. Structure of the PTH protein.
Ref:
1. Bieglmayer C, Prager G, Niederle B (October 2002). "Kinetic analyses of parathyroid hormone clearance as measured by three rapid immunoassays during parathyroidectomy". Clin. Chem. 48 (10): 1731–8.
- Pristimerin
Catalog No.:BCN2315
CAS No.:1258-84-0
- CEP-33779
Catalog No.:BCC2199
CAS No.:1257704-57-6
- Amikacin hydrate
Catalog No.:BCC4621
CAS No.:1257517-67-1
- TBA354
Catalog No.:BCC6459
CAS No.:1257426-19-9
- TC-E 5006
Catalog No.:BCC7981
CAS No.:1257395-14-4
- GSK 789472 hydrochloride
Catalog No.:BCC7818
CAS No.:1257326-24-1
- NPEC-caged-dopamine
Catalog No.:BCC7837
CAS No.:1257326-23-0
- NPEC-caged-serotonin
Catalog No.:BCC7836
CAS No.:1257326-22-9
- NPEC-caged-(S)-3,4-DCPG
Catalog No.:BCC7652
CAS No.:1257323-85-5
- NPEC-caged-(S)-AMPA
Catalog No.:BCC7789
CAS No.:1257323-84-4
- NPEC-caged-noradrenalin
Catalog No.:BCC7835
CAS No.:1257323-83-3
- TC-G 24
Catalog No.:BCC6146
CAS No.:1257256-44-2
- Deacetylorientalide
Catalog No.:BCN7310
CAS No.:1258517-59-7
- 9-O-Ethyldeacetylorientalide
Catalog No.:BCN7311
CAS No.:1258517-60-0
- LY2940680
Catalog No.:BCC3935
CAS No.:1258861-20-9
- (+)-Glaucarubinone
Catalog No.:BCN7956
CAS No.:1259-86-5
- 24-Methylenecycloartanol acetate
Catalog No.:BCN6137
CAS No.:1259-94-5
- SAMS Peptide
Catalog No.:BCC5745
CAS No.:125911-68-4
- JP 1302 dihydrochloride
Catalog No.:BCC7449
CAS No.:1259314-65-2
- VU 0155041 sodium salt
Catalog No.:BCC7642
CAS No.:1259372-69-4
- SCH 23390 hydrochloride
Catalog No.:BCC6849
CAS No.:125941-87-9
- 10-O-Ethylcannabitriol
Catalog No.:BCN7312
CAS No.:1259515-25-7
- Griseofulvin
Catalog No.:BCC5327
CAS No.:126-07-8
- Solasodine
Catalog No.:BCN2346
CAS No.:126-17-0
A comparison of the anabolic effects of rat and bovine parathyroid hormone (1-34) in ovariectomized rats.[Pubmed:15758479]
J Musculoskelet Neuronal Interact. 2001 Sep;2(1):77-83.
The current study was designed to compare the skeletal effects of comparable doses of rat parathyroid hormone 1-34 (rPTH) and bovine parathyroid hormone 1-34 (bPTH) in ovariectomized (OVX) rats. Female Sprague-Dawley rats were OVX or sham-operated at 6 months of age and maintained untreated for 28 days after surgery. Baseline control and OVX rats were sacrificed at the beginning of treatment. Beginning 28 days post-OVX, the remaining rats were subcutaneously injected daily with rPTH or bPTH at 0, 5, 25, or 50 microg/kg/d for 28 days. Bone area, bone mineral content (BMC), and bone mineral density (BMD) of the distal femoral metaphyses were determined ex vivo using dual energy X-ray absorptiometry. Quantitative bone histomorphometry was performed on undecalcified longitudinal sections of the proximal tibia from each rat. Baseline OVX rats exhibited osteopenia as demonstrated by their significantly reduced femoral BMD and proximal tibia cancellous bone volume compared with those of baseline sham controls. Both rPTH and bPTH restored bone in OVX rats by markedly stimulating bone formation in a dose-dependent manner. However, a difference in potency between the two forms of PTH was evident. The percentage increases of BMC, BMD, cancellous bone volume, trabecular thickness, mineralizing surface, and bone formation rate in the OVX rats treated with bPTH at 5 microg/kg/d were the same as or above those treated with rPTH at the 25 microg/kg/d dose level. A relative potency analysis showed that bPTH was approximately 4- to 6-fold relatively more potent than rPTH in increasing distal femoral BMD as well as cancellous bone volume, mineralizing surface, and bone formation rate of proximal tibial metaphyses at comparable dose levels and a given time. These results may serve as a reference for in vivo study design when rPTH or bPTH are to be the agents for studies on bone anabolism.
Solution structures of human parathyroid hormone fragments hPTH(1-34) and hPTH(1-39) and bovine parathyroid hormone fragment bPTH(1-37).[Pubmed:10623601]
Biochem Biophys Res Commun. 2000 Jan 7;267(1):213-20.
Parathyroid hormone (PTH) is involved in regulation of the calcium level in blood and has an influence on bone metabolism, thus playing a role in osteoporosis therapy. In this study, the structures of the human PTH fragments (1-34) and (1-39) as well as bovine PTH(1-37) in aqueous buffer solution under near physiological conditions were determined using two-dimensional nuclear magnetic resonance spectroscopy. The overall structure of the first 34 amino acids of these three peptides is virtually identical, exhibiting a short NH(2)-terminal and a longer COOH-terminal helix as well as a defined loop region from His14 to Ser17, stabilized by hydrophobic interactions. bPTH(1-37), which has a higher biological activity, shows a better-defined NH(2)-terminal part. In contrast to NH(2)-terminal truncations, which cause destabilization of helical structure, neither COOH-terminal truncation nor elongation significantly influences the secondary structure. Furthermore, we investigated the structure of hPTH(1-34) in 20% trifluoroethanol solution. In addition to its helix-stabilizing effect, trifluorethanol causes the loss of tertiary hydrophobic interactions.
Structure-function relationship studies of bovine parathyroid hormone [bPTH(1-34)] analogues containing alpha-amino-iso-butyric acid (Aib) residues.[Pubmed:12601801]
Biopolymers. 2003 Mar;68(3):437-57.
The N-terminal 1-34 fragments of the parathyroid hormone (PTH) and parathyroid hormone-related protein (PTHrP) elicit the full spectrum of bone-related biological activities of the intact native sequences. It has been suggested that the structural elements essential for bioactivity are two helical segments located at the N-terminal and C-terminal sequences, connected by hinges or flexible points around positions 12 and 19. In order to assess the relevance of the local conformation around Gly(12) upon biological function, we synthesized and characterized the following PTH(1-34) analogues containing Aib residues: (I) A-V-S-E-I-Q-F-nL-H-N-Aib-G-K-H-L-S-S-nL-E-R-V-E-Nal-L-R-K-K-L-Q-D-V-H-N-Y-NH(2) ([Nle(8,18), Aib(11), Nal(23),Tyr(34)]bPTH(1-34)-NH(2)); (II) A-V-S-E-I-Q-F-nL-H-N-L-Aib-K-H-L-S-S-nL-E-R-V-E-Nal-L-R-K-K-L-Q-D-V-H-N-Y-NH(2) ([Nle(8,18), Aib(12),Nal(23),Tyr(34)]bPTH(1-34)-NH(2)); (III) A-V-S-E-I-Q-F-nL-H-N-L-G-Aib-H-L-S-S-nL-E-R-V-E-Nal-L-R-K-K-L-Q-D-V-H-N-Y-NH(2) ([Nle(8,18), Aib(13), Nal(23),Tyr(34)]bPTH(1-34)-NH(2)); (IV) A-V-S-E-I-Q-F-nL-H-N-Aib-Aib-K-H-L-S-S-nL-E-R-V-E-Nal-L-R-K-K-L-Q-D-V-H-N-YNH(2) ([Nle(8,18), Aib(11,12), Nal(23),Tyr(34)]bPTH(1-34)-NH(2)); (V) A-V-S-E-I-Q-F-nL-H-N-L-Aib-Aib-H-L-S-S-nL-E-R-V-E-Nal-L-R-K-K-L-Q-D-V-H-N-Y-NH(2) ([Nle(8,18), Aib(12,13),Nal(23),Tyr(34)]bPTH(1-34)-NH(2)). (nL= Nle; Nal= L-(2-naphthyl)-alanine; Aib= alpha-amino-isobutyric acid.) The introduction of Aib residues at position 11 in analogue I or at positions 11 and 12 in analogue IV resulted in a 5-20-fold lower efficacy and a substantial loss of binding affinity compared to the parent compound [Nle(8,18), Nal(23),Tyr(34)]bPTH(1-34)-NH(2). Both binding affinity and adenylyl cyclase stimulation activity are largely restored when the Aib residues are introduced at position 12 in analogue II, 13 in analogue III, and 12-13 in analogue V. The conformational properties of the analogues in aqueous solution containing dodecylphosphocholine micelles were studied by CD, two-dimensional (2D) NMR and computer simulations. The results indicated the presence of two helical segments in all analogues, located at the N-terminal and C-terminal sequences. Insertion of Aib residues at positions 12 and 13, or of Aib dyads at positions 11-12 and 12-13, enhances the stability of the N-terminal helix of all analogues. In all analogues the Aib residues are included in the helical segments. These results confirmed the importance of the helical structure in the N-terminal activation domain, as well as of the presence of the Leu(11) hydrophobic side chain in the native sequence, for PTH-like bioactivity.
Modulation of osteogenic cell ultrastructure by RS-23581, an analog of human parathyroid hormone (PTH)-related peptide-(1-34), and bovine PTH-(1-34).[Pubmed:7628402]
Endocrinology. 1995 Aug;136(8):3624-31.
RS-23581, a synthetic analog of human PTH-related protein-(1-34), and the amino-terminal 34 amino acids of bovine PTH [bPTH-(1-34)] increase bone mineral density. We wished to determine how quickly the ultrastructure of the osteogenic cells, i.e. osteoblasts and lining cells, of the cancellous bone of the second lumbar vertebra of ovariectomized rats was altered in response to the initiation and cessation of treatment. Ovariectomized rats were injected daily with 80 micrograms/kg RS-23581, bPTH-(1-34), or vehicle for 19 days. Animals were killed throughout the treatment period and during the ensuing 10 days. By 5 days after the initiation of treatment with either peptide, the cells on the trabecular surface were predominantly (> 90%) osteoblasts, with only a small increase in the total cell number. Throughout the dosing period, the relative area of the cytoplasm of osteogenic cells from rats treated with RS-23581 or bPTH-(1-34) was greater than that of cells from the ovariectomized control group, suggesting a relationship between bone formation and cytoplasmic mass. By 7 days after the cessation of treatment, the trabecular surface was covered predominantly by lining cells without a change in cell number. Thus, these peptides apparently promote the osteoblast phenotype; the osteoblasts revert to lining cells after the peptides are withdrawn.


