Parathyroid hormone (1-34) (human)Increases blood calcium level CAS# 52232-67-4 |
- Parathyroid Hormone (1-34), bovine
Catalog No.:BCC1040
CAS No.:12583-68-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 52232-67-4 | SDF | Download SDF |
| PubChem ID | 16129682 | Appearance | Powder |
| Formula | C181H291N55O51S2 | M.Wt | 4117.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Forteo; Parathyroid hormone (1-34); Teriparatidum [Latin]; Teriparatida [Spanish]; 1-34-Human PTH; Parathyroid hormone peptide (1-34) | ||
| Solubility | H2O : ≥ 50 mg/mL (12.14 mM) *"≥" means soluble, but saturation unknown. | ||
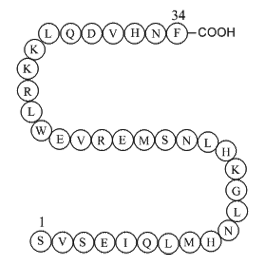
| Sequence | SVSEIQLMHNLGKHLNSMERVEWLRKKLQD | ||
| SMILES | CCC(C)C(C(=O)NC(CCC(=O)N)C(=O)NC(CC(C)C)C(=O)NC(CCSC)C(=O)NC(CC1=CNC=N1)C(=O)NC(CC(=O)N)C(=O)NC(CC(C)C)C(=O)NCC(=O)NC(CCCCN)C(=O)NC(CC2=CNC=N2)C(=O)NC(CC(C)C)C(=O)NC(CC(=O)N)C(=O)NC(CO)C(=O)NC(CCSC)C(=O)NC(CCC(=O)O)C(=O)NC(CCCNC(=N)N)C(=O)NC(C(C)C)C(=O)NC(CCC(=O)O)C(=O)NC(CC3=CNC4=CC=CC=C43)C(=O)NC(CC(C)C)C(=O)NC(CCCNC(=N)N)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)NC(CC(C)C)C(=O)NC(CCC(=O)N)C(=O)NC(CC(=O)O)C(=O)NC(C(C)C)C(=O)NC(CC5=CNC=N5)C(=O)NC(CC(=O)N)C(=O)NC(CC6=CC=CC=C6)C(=O)O)NC(=O)C(CCC(=O)O)NC(=O)C(CO)NC(=O)C(C(C)C)NC(=O)C(CO)N | ||
| Standard InChIKey | OGBMKVWORPGQRR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C181H291N55O51S2/c1-21-96(18)146(236-160(267)114(48-53-141(250)251)212-174(281)132(84-239)232-177(284)143(93(12)13)233-147(254)103(185)82-237)178(285)216-111(45-50-134(187)241)155(262)219-119(65-90(6)7)163(270)213-116(55-62-289-20)158(265)224-124(71-100-79-196-86-203-100)167(274)226-126(73-135(188)242)169(276)217-117(63-88(2)3)148(255)201-81-138(245)205-105(39-27-30-56-182)149(256)223-123(70-99-78-195-85-202-99)166(273)221-121(67-92(10)11)164(271)225-128(75-137(190)244)171(278)231-131(83-238)173(280)214-115(54-61-288-19)157(264)210-112(46-51-139(246)247)153(260)208-109(43-34-60-199-181(193)194)159(266)234-144(94(14)15)175(282)215-113(47-52-140(248)249)156(263)222-122(69-98-77-200-104-38-26-25-37-102(98)104)165(272)220-120(66-91(8)9)161(268)209-108(42-33-59-198-180(191)192)151(258)206-106(40-28-31-57-183)150(257)207-107(41-29-32-58-184)152(259)218-118(64-89(4)5)162(269)211-110(44-49-133(186)240)154(261)228-129(76-142(252)253)172(279)235-145(95(16)17)176(283)229-125(72-101-80-197-87-204-101)168(275)227-127(74-136(189)243)170(277)230-130(179(286)287)68-97-35-23-22-24-36-97/h22-26,35-38,77-80,85-96,103,105-132,143-146,200,237-239H,21,27-34,39-76,81-84,182-185H2,1-20H3,(H2,186,240)(H2,187,241)(H2,188,242)(H2,189,243)(H2,190,244)(H,195,202)(H,196,203)(H,197,204)(H,201,255)(H,205,245)(H,206,258)(H,207,257)(H,208,260)(H,209,268)(H,210,264)(H,211,269)(H,212,281)(H,213,270)(H,214,280)(H,215,282)(H,216,285)(H,217,276)(H,218,259)(H,219,262)(H,220,272)(H,221,273)(H,222,263)(H,223,256)(H,224,265)(H,225,271)(H,226,274)(H,227,275)(H,228,261)(H,229,283)(H,230,277)(H,231,278)(H,232,284)(H,233,254)(H,234,266)(H,235,279)(H,236,267)(H,246,247)(H,248,249)(H,250,251)(H,252,253)(H,286,287)(H4,191,192,198)(H4,193,194,199) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Human parathyroid hormone (hPTH) peptide fragment; contains the 34 N-terminal residues of hPTH. Agonist at parathyroid 1 (PTH1) and parathyroid 2 (PTH2) receptors. |

Parathyroid hormone (1-34) (human) Dilution Calculator

Parathyroid hormone (1-34) (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Parathyroid hormone (1-34) (human), (C181H291N55O51S2), a peptide with the sequence H2N-SVSEIQLMHNLGKHLNSMERVEWLRKKLQDVHNF-OH, MW= 4117.72. Parathyroid hormone (PTH), parathormone or parathyrin, is secreted by the chief cells of the parathyroid glands as a polypeptide containing 84 amino acids. It acts to increase the concentration of calcium (Ca2+) in the blood, whereas calcitonin (a hormone produced by the parafollicular cells (C cells) of the thyroid gland) acts to decrease calcium concentration. PTH acts to increase the concentration of calcium in the blood by acting upon the parathyroid hormone 1 receptor and the parathyroid hormone 2 receptor(1). Parathyroid hormone regulates serum calcium, It enhances the release of calcium from the large reservoir contained in the bones(2). It enhances active reabsorption of calcium and magnesium from distal tubules and the thick ascending limb(3). It enhances the absorption of calcium in the intestine by increasing the production of activated vitamin D.
Figure1 Formula of Parathyroid hormone (1-34) (human)
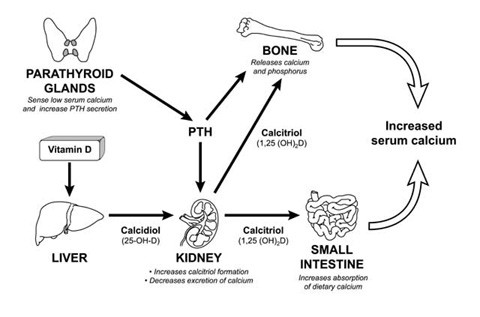
Figure2 The parathyroid hormone (PTH) System
Ref:
1. Bieglmayer C, Prager G, Niederle B (October 2002). "Kinetic analyses of parathyroid hormone clearance as measured by three rapid immunoassays during parathyroidectomy". Clin. Chem. 48 (10): 1731–8.
2. PooleK, Reeve J (2005). "Parathyroid hormone - a bone anabolic and catabolic agent". Curr Opin Pharmacol 5 (6): 612–7.
3. Coetzee M, Kruger MC (May 2004). "Osteoprotegerin-receptor activator of nuclear factor-kappaB ligand ratio: a new approach to osteoporosis treatment?". South. Med. J. 97 (5): 506–11.
- Kaempferol-4'-O-beta-D-glucopyranoside
Catalog No.:BCN8130
CAS No.:52222-74-9
- Ciprofibrate
Catalog No.:BCC2266
CAS No.:52214-84-3
- 3-Epicorosolic acid
Catalog No.:BCN5666
CAS No.:52213-27-1
- Lamalbid
Catalog No.:BCN3750
CAS No.:52212-87-0
- Tetrahydroberberine
Catalog No.:BCN2648
CAS No.:522-97-4
- Allo-Yohimbine
Catalog No.:BCN3487
CAS No.:522-94-1
- Dequalinium Chloride
Catalog No.:BCC4998
CAS No.:522-51-0
- Tetrahydrozoline HCl
Catalog No.:BCC4339
CAS No.:522-48-5
- Lochnerine
Catalog No.:BCN5667
CAS No.:522-47-4
- Norsanguinarine
Catalog No.:BCN3714
CAS No.:522-30-5
- Deguelin
Catalog No.:BCN4804
CAS No.:522-17-8
- Quercitrin
Catalog No.:BCN5665
CAS No.:522-12-3
- Isomucronulatol
Catalog No.:BCN1428
CAS No.:52250-35-8
- CGP 57380
Catalog No.:BCC5279
CAS No.:522629-08-9
- 3,5-Diprenyl-4-hydroxybenzaldehyde
Catalog No.:BCN4624
CAS No.:52275-04-4
- Ginsenoside Rf
Catalog No.:BCN1075
CAS No.:52286-58-5
- Ginsenoside Re
Catalog No.:BCN1073
CAS No.:52286-59-6
- Ginsenoside Rg2
Catalog No.:BCN1067
CAS No.:52286-74-5
- 4-Amino-2,5-dimethoxy-N-phenylbenzenesulphonamide
Catalog No.:BCC8676
CAS No.:52298-44-9
- Angelicin
Catalog No.:BCN5669
CAS No.:523-50-2
- Evolitrine
Catalog No.:BCN8350
CAS No.:523-66-0
- Flavoglaucin
Catalog No.:BCN6398
CAS No.:523-73-9
- Anisatin
Catalog No.:BCC8118
CAS No.:5230-87-5
- Vindorosine
Catalog No.:BCN5668
CAS No.:5231-60-7
Parathyroid hormone 1-34 reduces dexamethasone-induced terminal differentiation in human articular chondrocytes.[Pubmed:27608943]
Toxicology. 2016 Aug 10;368-369:116-128.
Intra-articular injection of dexamethasone (Dex) is occasionally used to relieve pain and inflammation in osteoarthritis (OA) patients. Dex induces terminal differentiation of chondrogenic mesenchymal stem cells in vitro and causes impaired longitudinal skeletal growth in vivo. Parathyroid hormone 1-34 (PTH 1-34) has been shown to reverse terminal differentiation of osteoarthritic articular chondrocytes. We hypothesized that Dex induces terminal differentiation of articular chondrocytes and that this effect can be mitigated by PTH 1-34 treatment. We tested the effect of Dex on terminal differentiation in human articular chondrocytes and further tested if PTH 1-34 reverses the effects. We found that Dex treatment downregulated chondrogenic-induced expressions of SOX-9, collagen type IIa1 (Col2a1), and aggrecan and reduced synthesis of cartilaginous matrix (Col2a1 and sulfated glycosaminoglycan) synthesis. Dex treatment upregulated chondrocyte hypertrophic markers of collagen type X and alkaline phosphatase at mRNA and protein levels, and it increased the cell size of articular chondrocytes and induced cell death. These results indicated that Dex induces terminal differentiation of articular chondrocytes. To test whether PTH 1-34 treatment reverses Dex-induced terminal differentiation of articular chondrocytes, PTH 1-34 was co-administered with Dex. Results showed that PTH 1-34 treatment reversed both changes of chondrogenic and hypertrophic markers in chondrocytes induced by Dex. PTH 1-34 also decreased Dex-induced cell death. PTH 1-34 treatment reduces Dex-induced terminal differentiation and apoptosis of articular chondrocytes, and PTH 1-34 treatment may protect articular cartilage from further damage when received Dex administration.
Effects of raloxifene and alendronate on non-enzymatic collagen cross-links and bone strength in ovariectomized rabbits in sequential treatments after daily human parathyroid hormone (1-34) administration.[Pubmed:27796444]
Osteoporos Int. 2017 Mar;28(3):1109-1119.
This study investigated the effects of raloxifene and alendronate to follow parathyroid hormone (PTH) on bone collagen and biomechanical properties in ovariectomized rabbits. Sequential treatments of raloxifene and alendronate after hPTH(1-34) treatment improved biomechanical properties with and without bone collagen improvement, respectively. INTRODUCTION: The standard sequential treatment to follow human parathyroid hormone (hPTH) (1-34) therapy for osteoporosis has yet to be determined. The objective of this study was to compare the effects of raloxifene and alendronate treatments to follow daily hPTH(1-34) treatment on non-enzymatic collagen cross-links, bone mass, and bone strength in ovariectomized (OVX) rabbits. METHODS: From 3 months after ovariectomy, seven month-old female New Zealand white rabbits were given either vehicle or hPTH(1-34) (8 mug/kg/day), once daily for 5 months. After hPTH(1-34) treatment, the hPTH(1-34)-treated animals were divided into two groups, and given raloxifene (10 mg/kg, daily) orally or alendronate (100 mug/kg, twice weekly) subcutaneously for 5 months. We evaluated bone mineral density (BMD), bone structural parameters, advanced glycation end product (AGE) content in collagen, and bone mechanical parameters including intrinsic parameters in the femur. RESULTS: Raloxifene (hPTH/RLX) and alendronate (hPTH/ALN) to follow hPTH(1-34) increased cortical thickness, maximum load, and maximum stress and decreased endocortical surface in the diaphysis, in addition to increasing total BMD in the distal metaphysis. Decreased trabecular AGE, pentosidine, and homocysteine contents and increased toughness and breaking energy were noted with hPTH/RLX treatment only. With hPTH/ALN treatment, no effects on non-enzymatic collagen cross-link AGEs were noted although increases in stiffness and elastic modulus were observed. CONCLUSION: These results suggest that sequential treatments with hPTH(1-34) and antiresorptive drugs (raloxifene and alendronate) have a beneficial effect on bone mass and biomechanical properties in OVX rabbits.
[Effect of recombinant human parathyroid hormone 1-34 on mandibular distraction osteogenesis in rabbits].[Pubmed:27055326]
Zhonghua Zheng Xing Wai Ke Za Zhi. 2015 Nov;31(6):450-5.
OBJECTIVE: To explore the effect of recombinant human parathyroid hormone 1-34 [rhPTH(1-34)] on bone regeneration rabbit mandible during distraction osteogenesis (DO). METHODS: 40 Japanese white rabbit (weight 2.0-2.5 kg) were randomly divided into control group and groups. The experimental groups were divided inito 12.5, 25 and 50 microg/kg group according to the dosage of rhPTH (1-34) in each group. Each group involved 10 rabbits, and unilateral DO models were established at the right mandible of the rabbits. From the first day of distraction to the day of execution, the rabbits in the experimental groups were injected subcutaneously rhPTH (1-34) of the corresponding dose respectively, and the rabbits in the control group were injected subcutaneously 2% heat inactivated rabbit serum 1 ml respectively.. Five rabbits in each group were executed respectively at 1 week and 3 weeks after completion of distraction, and the specimens of DO were harvested. The gross observation, X-ray examination, and histological study were performed. RESULTS: Gross appearance: At the first week of consolidation, the dense and opaque white tissue was seen in the distraction gap of the 50 microg/kg group, and the white translucent tissue was seen in the distraction gaps of the rest groups. At the third week of consolidation, the greyish white tissue was seen in the distraction gap of the control group, while the cartilage-like tissue was seen in the buccal side of the distraction gap of the 12.5 microg/kg group, the color of new-formed tissues was close to that of normal bone tissue in the lingual side. The buccal tissue at the edge of the distraction gap of the 25 microg/kg group fitted together with the primary bone tissue in its two sides. It was difficult to distinguish the boundaries between the distraction gap and the bone tissues in its two sides in the 50 microg/kg group. X-ray findings: At the first week of consolidation, a sparse opaque image was seen in the distraction gap of the 50 microg/kg group, and a low-density image was seen in the distraction gap of the rest groups. At the third week of consolidation, a sparse bone image was seen in the control group, and the edge of the bone was not continuous. With the increase of the dose in the experimental groups, the image of the distraction gap became more and more opaque, and the image of the distraction gap in the 50 microg/kg group was close to that of the normal bone tissue. HISTOLOGICAL FINDINGS: At the first week of consolidation, few osteoblasts were present at the edge of the distraction gap of the control group. A large number of bone cells and bone trabecular were present in the distraction gap of the 12.5 microg/kg group, the network of the bone trabecula was present in the 25 microg/kg group, and a few new bones were found in the 50 microg/kg group. At the third week of consolidation, the network of the trabecular bone was present in the distraction gap of the control group, while the network of the bone trabecula was present in the 12.5 microg/kg group, a lot of bone-like tissues in the 25 microg/kg group, and near-mature bone in the 50 microg/kg group. CONCLUSIONS: rhPTH(1-34) can promote the formation of new bone in the distracted gap during mandibular DO in rabbits.
Effects of Intermittent Administration of Parathyroid Hormone (1-34) on Bone Differentiation in Stromal Precursor Antigen-1 Positive Human Periodontal Ligament Stem Cells.[Pubmed:27069479]
Stem Cells Int. 2016;2016:4027542.
Periodontitis is the most common cause of tooth loss and bone destruction in adults worldwide. Human periodontal ligament stem cells (hPDLSCs) may represent promising new therapeutic biomaterials for tissue engineering applications. Stromal precursor antigen-1 (STRO-1) has been shown to have roles in adherence, proliferation, and multipotency. Parathyroid hormone (PTH) has been shown to enhance proliferation in osteoblasts. Therefore, in this study, we aimed to compare the functions of STRO-1(+) and STRO-1(-) hPDLSCs and to investigate the effects of PTH on the osteogenic capacity of STRO-1(+) hPDLSCs in order to evaluate their potential applications in the treatment of periodontitis. Our data showed that STRO-1(+) hPDLSCs expressed higher levels of the PTH-1 receptor (PTH1R) than STRO-1(-) hPDLSCs. In addition, intermittent PTH treatment enhanced the expression of PTH1R and osteogenesis-related genes in STRO-1(+) hPDLSCs. PTH-treated cells also exhibited increased alkaline phosphatase activity and mineralization ability. Therefore, STRO-1(+) hPDLSCs represented a more promising cell resource for biomaterials and tissue engineering applications. Intermittent PTH treatment improved the capacity for STRO-1(+) hPDLSCs to repair damaged tissue and ameliorate the symptoms of periodontitis.
Human parathyroid hormone (1-34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys.[Pubmed:17369013]
Bone. 2007 Jun;40(6):1475-82.
Several studies in rats have demonstrated that parathyroid hormone accelerates fracture healing by increasing callus formation or stimulating callus remodeling. However the effect of PTH on fracture healing has not been tested using large animals with Haversian remodeling system. Using cynomolgus monkey that has intracortical remodeling similar to humans, we examined whether intermittent treatment with human parathyroid hormone [hPTH(1-34)] accelerates the fracture healing process, especially callus remodeling, and restores geometrical shapes and mechanical properties of osteotomized bone. Seventeen female cynomolgus monkeys aged 18-19 years were allocated into three groups: control (CNT, n=6), low-dose PTH (0.75 microg/kg; PTH-L, n=6), and high-dose PTH (7.5 microg/kg; PTH-H, n=5) groups. In all animals, twice a week subcutaneous injection was given for 3 weeks. Then fracture was produced surgically by transversely cutting the midshaft of the right femur and fixing with stainless plate. After fracture, intermittent PTH treatment was continued until sacrifice at 26 weeks after surgery. The femora were assessed by soft X-ray, three-point bending mechanical test, histomorphometry, and degree of mineralization in bone (DMB) measurement. Soft X-ray showed that complete bone union occurred in all groups, regardless of treatment. Ultimate stress and elastic modulus in fractured femur were significantly higher in PTH-H than in CNT. Total area and percent bone area of the femur were significantly lower in both PTH-L and PTH-H than in CNT. Callus porosity decreased dose-dependently following PTH treatment. Mean DMB of callus was significantly higher in PTH-H than in CNT or PTH-L. These results suggested that PTH decreased callus size and accelerated callus maturation in the fractured femora. PTH accelerates the natural fracture healing process by shrinking callus size and increasing degree of mineralization of the fracture callus, thereby restoring intrinsic material properties of osteotomized femur shaft in cynomolgus monkeys although there were no significant differences among the groups for structural parameters.
The effects of programmed administration of human parathyroid hormone fragment (1-34) on bone histomorphometry and serum chemistry in rats.[Pubmed:9348185]
Endocrinology. 1997 Nov;138(11):4607-12.
PTH treatment can result in dramatic increases in cancellous bone volume in normal and osteopenic rats. However, this potentially beneficial response is only observed after pulsatile treatment; continuous infusion of PTH leads to hypercalcemia and bone abnormalities. The purpose of these studies was to determine the optimal duration of the PTH pulses. A preliminary study revealed that human PTH-(1-34) (hPTH) is cleared from circulation within 6 h after sc administration of an anabolic dose of the hormone (80 microg/kg). To establish the effects of gradually extending the duration of exposure to hPTH without increasing the daily dose, we programmed implanted Alzet osmotic pumps to deliver the 80 microg/kg x day dose of the hormone during pulses of 1, 2, and 6 h/day, or 40 microg/kg x day continuously. Discontinuous infusion was accomplished by alternate spacing of external tubing with hPTH solution and sesame oil. After 6 days of treatment, we evaluated serum chemistry and bone histomorphometry. As negative and positive controls, groups of rats received pumps that delivered vehicle only and 80 microg/kg x day hPTH by daily sc injection, respectively. Dynamic and static bone histomorphometry revealed that the daily sc injection and 1 h/day infusion dramatically increased osteoblast number and bone formation in the proximal tibial metaphysis, whereas longer infusion resulted in systemic side-effects, including up to a 10% loss in body weight, hypercalcemia, and histological changes in the proximal tibia resembling abnormalities observed in patients with chronic primary hyperparathyroidism, including peritrabecular marrow fibrosis and focal bone resorption. Infusion for as little as 2 h/day resulted in minor weight loss and changes in bone histology that were intermediate between sc and continuous administration. The results demonstrate that the therapeutic interval for hPTH exposure is brief, but that programmed administration of implanted hormone is a feasible alternative to daily injection as a route for administration of the hormone.


