TetrahydroberberineCAS# 522-97-4 |
- Canadine
Catalog No.:BCN5626
CAS No.:5096-57-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 522-97-4 | SDF | Download SDF |
| PubChem ID | 34458 | Appearance | Yellow powder |
| Formula | C20H21O4N | M.Wt | 339.38 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Tetrahydroberberine; Tetrahydroumbellatine; Xanthopuccine | ||
| Solubility | DMSO : 25 mg/mL (73.66 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
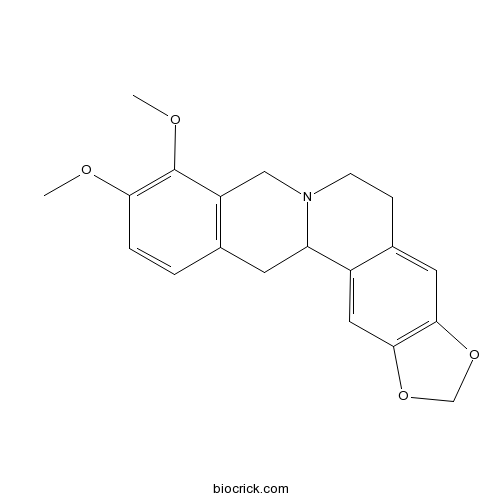
| SMILES | COC1=C(C2=C(CC3C4=CC5=C(C=C4CCN3C2)OCO5)C=C1)OC | ||
| Standard InChIKey | VZTUIEROBZXUFA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H21NO4/c1-22-17-4-3-12-7-16-14-9-19-18(24-11-25-19)8-13(14)5-6-21(16)10-15(12)20(17)23-2/h3-4,8-9,16H,5-7,10-11H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tetrahydroberberine, with D(2) receptor antagonist and 5-HT(1A) receptor agonist properties, has significant potential as a therapeutic for treatment of FD; it has antidopaminergic effect, and other pharmacological action on the central nervous system. Tetrahydroberberine can inhibit the rabbit platelet aggregation. |
| Targets | 5-HT Receptor | Dopamine Receptor |
| In vitro | Tetrahydroberberine, a pharmacologically active naturally occurring alkaloid.[Pubmed: 25836282]Acta Crystallogr C Struct Chem. 2015 Apr;71(Pt 4):262-5.
|
| In vivo | Tetrahydroberberine, an isoquinoline alkaloid isolated from corydalis tuber, enhances gastrointestinal motor function.[Pubmed: 21659472]J Pharmacol Exp Ther. 2011 Sep;338(3):917-24.
|
| Kinase Assay | Tetrahydroberberine suppresses dopamine-induced potassium current in acutely dissociated CA1 pyramidal neurons from rat hippocampus.[Pubmed: 8728473]Neurosci Lett. 1996 Apr 5;207(3):155-8.
|
| Animal Research | Inhibitory effect of tetrahydroberberine on platelet aggregation and thrombosis.[Pubmed: 8010106]Zhongguo Yao Li Xue Bao. 1994 Mar;15(2):133-5.
|

Tetrahydroberberine Dilution Calculator

Tetrahydroberberine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9465 mL | 14.7327 mL | 29.4655 mL | 58.931 mL | 73.6637 mL |
| 5 mM | 0.5893 mL | 2.9465 mL | 5.8931 mL | 11.7862 mL | 14.7327 mL |
| 10 mM | 0.2947 mL | 1.4733 mL | 2.9465 mL | 5.8931 mL | 7.3664 mL |
| 50 mM | 0.0589 mL | 0.2947 mL | 0.5893 mL | 1.1786 mL | 1.4733 mL |
| 100 mM | 0.0295 mL | 0.1473 mL | 0.2947 mL | 0.5893 mL | 0.7366 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Allo-Yohimbine
Catalog No.:BCN3487
CAS No.:522-94-1
- Dequalinium Chloride
Catalog No.:BCC4998
CAS No.:522-51-0
- Tetrahydrozoline HCl
Catalog No.:BCC4339
CAS No.:522-48-5
- Lochnerine
Catalog No.:BCN5667
CAS No.:522-47-4
- Norsanguinarine
Catalog No.:BCN3714
CAS No.:522-30-5
- Deguelin
Catalog No.:BCN4804
CAS No.:522-17-8
- Quercitrin
Catalog No.:BCN5665
CAS No.:522-12-3
- Evoxine
Catalog No.:BCN5664
CAS No.:522-11-2
- N'-Methylammodendrine
Catalog No.:BCN2147
CAS No.:52196-10-8
- 7-Hydroxy-2,3,4,5-tetrahydro-1H-benzofuro[2,3-c]azepin-1-one
Catalog No.:BCC3960
CAS No.:521937-07-5
- Piperitol
Catalog No.:BCN3968
CAS No.:52151-92-5
- H-Tyr(Bzl)-OBzl.HCl
Catalog No.:BCC3131
CAS No.:52142-01-5
- Lamalbid
Catalog No.:BCN3750
CAS No.:52212-87-0
- 3-Epicorosolic acid
Catalog No.:BCN5666
CAS No.:52213-27-1
- Ciprofibrate
Catalog No.:BCC2266
CAS No.:52214-84-3
- Kaempferol-4'-O-beta-D-glucopyranoside
Catalog No.:BCN8130
CAS No.:52222-74-9
- Parathyroid hormone (1-34) (human)
Catalog No.:BCC1046
CAS No.:52232-67-4
- Isomucronulatol
Catalog No.:BCN1428
CAS No.:52250-35-8
- CGP 57380
Catalog No.:BCC5279
CAS No.:522629-08-9
- 3,5-Diprenyl-4-hydroxybenzaldehyde
Catalog No.:BCN4624
CAS No.:52275-04-4
- Ginsenoside Rf
Catalog No.:BCN1075
CAS No.:52286-58-5
- Ginsenoside Re
Catalog No.:BCN1073
CAS No.:52286-59-6
- Ginsenoside Rg2
Catalog No.:BCN1067
CAS No.:52286-74-5
- 4-Amino-2,5-dimethoxy-N-phenylbenzenesulphonamide
Catalog No.:BCC8676
CAS No.:52298-44-9
Tetrahydroberberine, an isoquinoline alkaloid isolated from corydalis tuber, enhances gastrointestinal motor function.[Pubmed:21659472]
J Pharmacol Exp Ther. 2011 Sep;338(3):917-24.
Because delayed gastric emptying and impaired gastric accommodation are regarded as pathophysiological mechanisms underlying functional dyspepsia (FD), prokinetics and fundic relaxants have been suggested as a new treatment for FD. We isolated Tetrahydroberberine (THB), an isoquinoline alkaloid (5,8,13,13a-tetrahydro-9,10-dimethoxy-6H-benzo[g]-1,3-benzodioxolo[5,6-a]quinoliz ine) from Corydalis tuber, and found that it has micromolar affinity for dopamine D(2) (pK(i) = 6.08) and 5-HT(1A) (pK(i) = 5.38) receptors but moderate to no affinity for other relevant serotonin receptors (i.e., 5-HT(1B), 5-HT(1D), 5-HT(3), and 5-HT(4); pK(i) < 5.00). Oral administration of THB not only resulted in significantly accelerated gastric emptying of normal rats in a bell-shaped relationship, with a maximal efficacy at a dose of 30 mug/kg, but also restored the delayed gastric emptying caused by apomorphine, which might be mediated by an antidopaminergic effect. Data from electromyography indicated enhanced motor function of the upper gastrointestinal tract by THB, which occurred through strengthening contractility and shortening the contraction interval. Furthermore, in rats stressed by repeated restraint, a significantly higher shift in the pressure-volume curve by THB (10 mug/kg, p < 0.05), which was inhibited by [O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl)cyclo hexanecarboxamide trihydrochloride (WAY-100635), a 5-HT(1A) antagonist, and N(omega)-nitro-l-arginine methyl ester, a nitric-oxide synthase inhibitor but not a vasoactive intestinal peptide antagonist, was observed. Oral administration of THB resulted in a drastic increase of gastric accommodation in Beagle dogs. Area under the volume versus time curve was increased significantly by THB (30 mug/kg, p < 0.01) and comparable with that of sumatriptan (3 mg/kg), a potent fundic relaxant. Taken together, our data suggested that THB, with D(2) receptor antagonist and 5-HT(1A) receptor agonist properties, has significant potential as a therapeutic for treatment of FD.
Tetrahydroberberine suppresses dopamine-induced potassium current in acutely dissociated CA1 pyramidal neurons from rat hippocampus.[Pubmed:8728473]
Neurosci Lett. 1996 Apr 5;207(3):155-8.
Effects of Tetrahydroberberine (THB) on dopamine (DA)-induced response have been investigated in single pyramidal neurons freshly dissociated from CA1 area of the hippocampus using the nystatin perforated patch-clamp, whole-cell recording technique under voltage-clamp configuration. At a holding potential (VH) of -20mV, DA-induced responses included a transient outward current, a slow inward current and a combination of the two. The outward current had a reversal potential of -83.5 +/- 8.0 mV which was close to K+ equilibrium potential and was sensitive to TEA, suggesting that this outward current was carried by K+. Application of THB reversibly suppressed three type responses induced by DA with different degrees of inhibitory ratio. THB inhibited the DA-induced outward current in a concentration-dependent manner with an IC50 of 13 microM. The maximal value of the concentration-response curve for DA-induced outward current was suppressed by THB, suggesting a non-competitive inhibition. The results support the hypothesis that THB acts as a novel dopaminergic system antagonist. Furthermore, THB inhibits the DA-induced K+ current non-competitively in single dissociated cells, implying that THB may exhibit other pharmacological action on the central nervous system.
Tetrahydroberberine, a pharmacologically active naturally occurring alkaloid.[Pubmed:25836282]
Acta Crystallogr C Struct Chem. 2015 Apr;71(Pt 4):262-5.
Tetrahydroberberine (systematic name: 9,10-dimethoxy-5,8,13,13a-tetrahydro-6H-benzo[g][1,3]benzodioxolo[5,6-a]quinolizi ne), C20H21NO4, a widely distributed naturally occurring alkaloid, has been crystallized as a racemic mixture about an inversion center. A bent conformation of the molecule is observed, with an angle of 24.72 (5) degrees between the arene rings at the two ends of the reduced quinolizinium core. The intermolecular hydrogen bonds that play an apparent role in crystal packing are 1,3-benzodioxole -CH2...OCH3 and -OCH3...OCH3 interactions between neighboring molecules.
Inhibitory effect of tetrahydroberberine on platelet aggregation and thrombosis.[Pubmed:8010106]
Zhongguo Yao Li Xue Bao. 1994 Mar;15(2):133-5.
Tetrahydroberberine (THB), an alkaloid extracted from Corydalis ambigua, inhibited the rabbit platelet aggregation triggered by arachidonic acid (AA), ADP, and collagen with IC50 of 0.86, 1.31, and 1.10 mmol.L-1, respectively. THB reduced the thromboxane B2 (TXB2) generation in rabbit platelet-rich plasma triggered by AA. THB 30 mg.kg-1.d-1 ip for 3 or 5 d restrained the ADP-induced platelet aggregation in rats. THB 30 mg.kg-1.d-1 ip for 1, 3, or 5 d inhibited the AA-induced platelet aggregation in rats. THB 15-30 mg.kg-1 iv showed an inhibition of venous thrombosis in rats. The results show that THB is a potent inhibitor of platelet aggregation in vitro and in vivo and is a promising antithrombotic drug.


