LochnerineCAS# 522-47-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 522-47-4 | SDF | Download SDF |
| PubChem ID | 6436184 | Appearance | Powder |
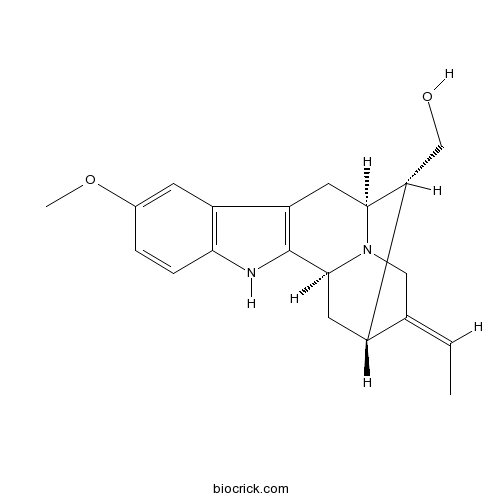
| Formula | C20H24N2O2 | M.Wt | 324.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC=C1CN2C3CC1C(C2CC4=C3NC5=C4C=C(C=C5)OC)CO | ||
| Standard InChIKey | YTIVOMMYBBBYFH-BOPWJTLDSA-N | ||
| Standard InChI | InChI=1S/C20H24N2O2/c1-3-11-9-22-18-8-15-14-6-12(24-2)4-5-17(14)21-20(15)19(22)7-13(11)16(18)10-23/h3-6,13,16,18-19,21,23H,7-10H2,1-2H3/b11-3-/t13-,16+,18-,19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Lochnerine shows potent vasorelaxant activity. 2. Lochnerine shows some antitumor activity, it can bring about complete inhibition of cell growth in P388 leukemia cells in vitro. |
| Targets | NO | Calcium Channel |

Lochnerine Dilution Calculator

Lochnerine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0826 mL | 15.4131 mL | 30.8261 mL | 61.6523 mL | 77.0654 mL |
| 5 mM | 0.6165 mL | 3.0826 mL | 6.1652 mL | 12.3305 mL | 15.4131 mL |
| 10 mM | 0.3083 mL | 1.5413 mL | 3.0826 mL | 6.1652 mL | 7.7065 mL |
| 50 mM | 0.0617 mL | 0.3083 mL | 0.6165 mL | 1.233 mL | 1.5413 mL |
| 100 mM | 0.0308 mL | 0.1541 mL | 0.3083 mL | 0.6165 mL | 0.7707 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Norsanguinarine
Catalog No.:BCN3714
CAS No.:522-30-5
- Deguelin
Catalog No.:BCN4804
CAS No.:522-17-8
- Quercitrin
Catalog No.:BCN5665
CAS No.:522-12-3
- Evoxine
Catalog No.:BCN5664
CAS No.:522-11-2
- N'-Methylammodendrine
Catalog No.:BCN2147
CAS No.:52196-10-8
- 7-Hydroxy-2,3,4,5-tetrahydro-1H-benzofuro[2,3-c]azepin-1-one
Catalog No.:BCC3960
CAS No.:521937-07-5
- Piperitol
Catalog No.:BCN3968
CAS No.:52151-92-5
- H-Tyr(Bzl)-OBzl.HCl
Catalog No.:BCC3131
CAS No.:52142-01-5
- 3-O-Acetylpinobanksin
Catalog No.:BCN5660
CAS No.:52117-69-8
- 2,4-Dihydroxy-6-methoxy-3-formylacetophenone
Catalog No.:BCN1430
CAS No.:52117-67-6
- Karanjin
Catalog No.:BCN8370
CAS No.:521-88-0
- Broxyquinoline
Catalog No.:BCC4642
CAS No.:521-74-4
- Tetrahydrozoline HCl
Catalog No.:BCC4339
CAS No.:522-48-5
- Dequalinium Chloride
Catalog No.:BCC4998
CAS No.:522-51-0
- Allo-Yohimbine
Catalog No.:BCN3487
CAS No.:522-94-1
- Tetrahydroberberine
Catalog No.:BCN2648
CAS No.:522-97-4
- Lamalbid
Catalog No.:BCN3750
CAS No.:52212-87-0
- 3-Epicorosolic acid
Catalog No.:BCN5666
CAS No.:52213-27-1
- Ciprofibrate
Catalog No.:BCC2266
CAS No.:52214-84-3
- Kaempferol-4'-O-beta-D-glucopyranoside
Catalog No.:BCN8130
CAS No.:52222-74-9
- Parathyroid hormone (1-34) (human)
Catalog No.:BCC1046
CAS No.:52232-67-4
- Isomucronulatol
Catalog No.:BCN1428
CAS No.:52250-35-8
- CGP 57380
Catalog No.:BCC5279
CAS No.:522629-08-9
- 3,5-Diprenyl-4-hydroxybenzaldehyde
Catalog No.:BCN4624
CAS No.:52275-04-4
Vasorelaxant activity of indole alkaloids from Tabernaemontana dichotoma.[Pubmed:22350216]
J Nat Med. 2013 Jan;67(1):9-16.
The aim of this study was to search for bioactive natural products from medicinal plants targeting vasorelaxant activity and we found the methanol extract from bark of Tabernaemontana dichotoma showed vasorelaxant activity on rat aorta. We isolated eight indole alkaloids including 10-methoxyalstonerine (1), a new macroline type indole alkaloid, from bark of T. dichotoma. These were respectively identified as 10-methoxyaffinisine (2), Lochnerine (3), cathafoline (4), (-)-alstonerine (5), 19,20-dehydro-10-methoxytalcarpine (6), alstonisine (7), and alstonal (8) based on spectroscopic analysis. Among them, sarpagine type (2 and 3), akuammiline type (4), and macroline oxindole type (7 and 8) showed potent vasorelaxant activity. Mechanism of action on vasorelaxant activity of 10-methoxyaffinisine (2), cathafoline (4), and alstonisine (7) was clarified. Effects of 10-methoxyaffinisine (2), cathafoline (4), and alstonisine (7) were partially mediated the NO release from endothelial cells. Furthermore, 10-methoxyaffinisine (2) and alstonisine (7) attribute to the inhibitory effect of VDC and ROC, and cathafoline (4) have inhibitory effect on Ca(2+) influx via ROC. In addition, 10-methoxyaffinisine (2) as a major compound from bark of T. dichotoma showed hypotensive effect on normotensive rats in vivo.
Non-antitumor vinca alkaloids reverse multidrug resistance in P388 leukemia cells in vitro.[Pubmed:3082832]
Jpn J Cancer Res. 1986 Feb;77(2):197-204.
Twelve monomeric or dimeric alkaloids from Vinca rosea Linn., which had been reported to have little or no antitumor activity, were investigated to determine their combined effects with either vincristine or daunorubicin on in vitro cell growth of a P388 subline resistant to vincristine and cross-resistant to anthracyclines. We found that the combinations at subcytotoxic concentrations induced significant growth inhibition of the resistant cells, but not of the sensitive cells. Of the alkaloids examined, catharine, vindoline, catharanthine, vincarodine, and Lochnerine were able to bring about complete inhibition of cell growth. Further in vitro study using vindoline revealed that at 10 micrograms/ml it was able to completely reverse not only resistance to vincristine but also cross-resistance to vinblastine, daunorubicin, and adriamycin. In addition, we found that vinca alkaloids active in reversing resistance possess potent activities to enhance the net uptake of not only vincristine but also daunorubicin by the resistant cells, and this effect was proved to result from their inhibitory action on the active efflux process. These results provide further support for our hypothesis that both anthracyclines and vinca alkaloids can inhibit their own efflux process by interacting with the cell membrane, and this similarity provides a basis for their reciprocal cross-resistance, irrespective of their different chemical structures.
Stereospecific approach to the synthesis of ring-A oxygenated sarpagine indole alkaloids. Total synthesis of the dimeric indole alkaloid P-(+)-dispegatrine and six other monomeric indole alkaloids.[Pubmed:23721107]
J Org Chem. 2013 Jul 5;78(13):6471-87.
The first regio- and stereocontrolled total synthesis of the bisphenolic, bisquaternary alkaloid (+)-dispegatrine (1) has been accomplished in an overall yield of 8.3% (12 reaction vessels) from 5-methoxy-d-tryptophan ethyl ester (17). A crucial late-stage thallium(III) mediated intermolecular oxidative dehydrodimerization was employed in the formation of the C9-C9' biaryl axis in 1. The complete stereocontrol observed in this key biaryl coupling step is due to the asymmetric induction by the natural sarpagine configuration of the monomer Lochnerine (6) and was confirmed by both the Suzuki and the oxidative dehydrodimerization model studies on the tetrahydro beta-carboline (35). The axial chirality of the Lochnerine dimer (40) and in turn dispegatrine (1) was established by X-ray crystallography and was determined to be P(S). Additionally, the first total synthesis of the monomeric indole alkaloids (+)-spegatrine (2), (+)-10-methoxyvellosimine (5), (+)-Lochnerine (6), lochvinerine (7), (+)-sarpagine (8), and (+)-lochneram (11) were also achieved via the common pentacyclic intermediate 16.


