QuercitrinCAS# 522-12-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

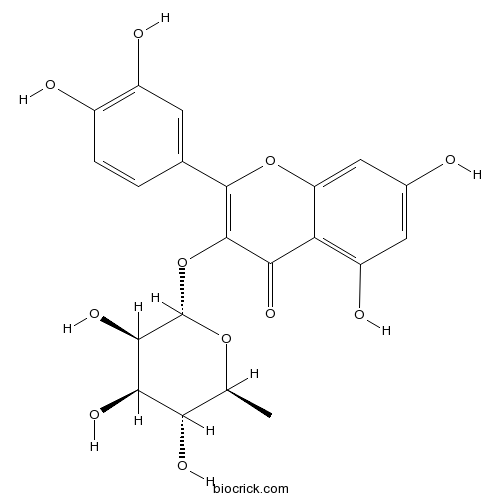
| Cas No. | 522-12-3 | SDF | Download SDF |
| PubChem ID | 5280459 | Appearance | Yellow powder |
| Formula | C21H20O11 | M.Wt | 448.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Quercetin 3-rhamnoside | ||
| Solubility | DMSO : ≥ 31 mg/mL (69.14 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one | ||
| SMILES | CC1C(C(C(C(O1)OC2=C(OC3=CC(=CC(=C3C2=O)O)O)C4=CC(=C(C=C4)O)O)O)O)O | ||
| Standard InChIKey | OXGUCUVFOIWWQJ-HQBVPOQASA-N | ||
| Standard InChI | InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Quercitrin is an antibacterial agent and inhibits the oxidation of low-density lipoproteins and prevent an allergic reaction; quercitrin and DNJ in combination as a potent anticariogenic agent against S. mutans.Quercitrin has antioxidant, anti-inflammatory, anti-cancer, and anti-allergic activities. Quercitrin has antiproliferative and apoptotic effects on lung cancer cells and colon cancer cells by modulating the immune response; it promotes osteoblast differentiation in MC3T3-E1 cells and also inhibit osteoclastogenesis in RAW264.7 cells. |
| Targets | Caspase | MMP(e.g.TIMP) | NF-kB | JNK | HO-1 | ERK | p38MAPK | PARP |
| In vitro | Molecular mechanisms of quercitrin-induced apoptosis in non-small cell lung cancer.[Pubmed: 25193878]Arch Med Res. 2014 Aug;45(6):445-54.Quercitrin (QR; quercetin-3-O-rhamnoside) has been used previously as an antibacterial agent and has been shown to inhibit the oxidation of low-density lipoproteins and prevent an allergic reaction. Furthermore, it was demonstrated that Quercitrin exerts protective effects against H2O2-induced dysfunction in lung fibroblast cells. However, the mechanisms of Quercitrin effects on cancer cell proliferation and apoptosis is not well understood. The aim of this study is to investigate the cytotoxic and apoptotic effects of Quercitrin and the molecular mechanisms of Quercitrin-induced apoptosis in non-small cell lung cancer (NSCLC) cell lines.
Inhibition of major virulence pathways of Streptococcus mutans by quercitrin and deoxynojirimycin: a synergistic approach of infection control.[Pubmed: 24622055]PLoS One. 2014 Mar 12;9(3):e91736.To evaluate the synergistic effect of Quercitrin and Deoxynojirimycin (DNJ) together with their individual inhibitory effect against virulence pathways of Streptococcus mutans.
|
| In vivo | In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway.[Pubmed: 15668926 ]Eur J Immunol. 2005 Feb;35(2):584-92.Quercetin is a common antioxidant flavonoid found in vegetables, which is usually present in glycosylated forms, such as Quercitrin (3-rhamnosylquercetin). Previous in vitro experiments have shown that quercetin exerts a bigger effect than Quercitrin in the down-regulation of the inflammatory response.
However, such results have not been reproduced in in vivo experimental models of intestinal inflammation, in which quercetin did not show beneficial effects while its glycosides, Quercitrin or rutin, have demonstrated their effectiveness.
|
| Kinase Assay | Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages.[Pubmed: 15876423]Biochem Pharmacol. 2005 Jun 15;69(12):1839-51.In the present study, we examine the protective mechanism of quercetin (QE) on oxidative stress-induced cytotoxic effect in RAW264.7 macrophages.
|
| Cell Research | Quercitrin and taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells.[Pubmed: 24060614]Apoptotic Effects of Quercitrin on DLD-1 Colon Cancer Cell Line.[Pubmed: 25096395]Pathol Oncol Res. 2015 Apr;21(2):333-8.Quercetin, which is the most abundant bioflavonoid compound, is mainly present in the glycoside form of Quercitrin. Although different studies indicated that Quercitrin is a potent antioxidant, the action of this compound is not well understood. In this study, we investigated whether Quercitrin has apoptotic and antiproliferative effects in DLD-1 colon cancer cell lines.
Biochem Pharmacol. 2013 Nov 15;86(10):1476-86.Flavonoids are natural antioxidants that positively influence bone metabolism. The present study screened among different flavonoids to identify biomolecules for potential use in bone regeneration. For this purpose, we used MC3T3-E1 and RAW264.7 cells to evaluate their effect on cell viability and cell differentiation.
|

Quercitrin Dilution Calculator

Quercitrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2302 mL | 11.1508 mL | 22.3015 mL | 44.603 mL | 55.7538 mL |
| 5 mM | 0.446 mL | 2.2302 mL | 4.4603 mL | 8.9206 mL | 11.1508 mL |
| 10 mM | 0.223 mL | 1.1151 mL | 2.2302 mL | 4.4603 mL | 5.5754 mL |
| 50 mM | 0.0446 mL | 0.223 mL | 0.446 mL | 0.8921 mL | 1.1151 mL |
| 100 mM | 0.0223 mL | 0.1115 mL | 0.223 mL | 0.446 mL | 0.5575 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Quercitrin is a natural compound found in Tartary buckwheat with a potential anti-inflammation effect that is used to treat heart and vascular conditions. IC50 value: Target: In vitro: There were significant increases in caspase-3 activity, loss of MMP, and increases in the apoptotic cell population in response to quercitrin in DLD-1 colon cancer cells in a time- and dose-dependent manner. [1] In vivo: ICR mice received CCl4 intraperitoneally with or without quercitrin co-administration for 4 weeks. Data showed that quercitrin significantly suppressed the elevation of reactive oxygen species (ROS) production and malondialdehyde (MDA) content, reduced tissue plasminogen activator (t-PA) activity, enhanced the antioxidant enzyme activities and abrogated cytochrome P450 2E1 (CYP2E1) induction in mouse brains. [2]
References:
[1]. Cincin ZB, et al. Apoptotic Effects of Quercitrin on DLD-1 Colon Cancer Cell Line. Pathol Oncol Res. 2015 Apr;21(2):333-8.
[2]. Ma JQ, et al. Quercitrin offers protection against brain injury in mice by inhibiting oxidative stress and inflammation. Food Funct. 2016 Jan 20;7(1):549-56.
[3]. Li W, et al. Quercitrin ameliorates the development of systemic lupus erythematosus-like disease in a chronic graft-versus-host murine model. Am J Physiol Renal Physiol. 2016 Feb 24:ajprenal.00249.2015.
- Evoxine
Catalog No.:BCN5664
CAS No.:522-11-2
- N'-Methylammodendrine
Catalog No.:BCN2147
CAS No.:52196-10-8
- 7-Hydroxy-2,3,4,5-tetrahydro-1H-benzofuro[2,3-c]azepin-1-one
Catalog No.:BCC3960
CAS No.:521937-07-5
- Piperitol
Catalog No.:BCN3968
CAS No.:52151-92-5
- H-Tyr(Bzl)-OBzl.HCl
Catalog No.:BCC3131
CAS No.:52142-01-5
- 3-O-Acetylpinobanksin
Catalog No.:BCN5660
CAS No.:52117-69-8
- 2,4-Dihydroxy-6-methoxy-3-formylacetophenone
Catalog No.:BCN1430
CAS No.:52117-67-6
- Karanjin
Catalog No.:BCN8370
CAS No.:521-88-0
- Broxyquinoline
Catalog No.:BCC4642
CAS No.:521-74-4
- Cinnamoylcocaine
Catalog No.:BCN1429
CAS No.:521-67-5
- Frangulin A
Catalog No.:BCC8174
CAS No.:521-62-0
- Physcion
Catalog No.:BCN5663
CAS No.:521-61-9
- Deguelin
Catalog No.:BCN4804
CAS No.:522-17-8
- Norsanguinarine
Catalog No.:BCN3714
CAS No.:522-30-5
- Lochnerine
Catalog No.:BCN5667
CAS No.:522-47-4
- Tetrahydrozoline HCl
Catalog No.:BCC4339
CAS No.:522-48-5
- Dequalinium Chloride
Catalog No.:BCC4998
CAS No.:522-51-0
- Allo-Yohimbine
Catalog No.:BCN3487
CAS No.:522-94-1
- Tetrahydroberberine
Catalog No.:BCN2648
CAS No.:522-97-4
- Lamalbid
Catalog No.:BCN3750
CAS No.:52212-87-0
- 3-Epicorosolic acid
Catalog No.:BCN5666
CAS No.:52213-27-1
- Ciprofibrate
Catalog No.:BCC2266
CAS No.:52214-84-3
- Kaempferol-4'-O-beta-D-glucopyranoside
Catalog No.:BCN8130
CAS No.:52222-74-9
- Parathyroid hormone (1-34) (human)
Catalog No.:BCC1046
CAS No.:52232-67-4
Apoptotic Effects of Quercitrin on DLD-1 Colon Cancer Cell Line.[Pubmed:25096395]
Pathol Oncol Res. 2015 Apr;21(2):333-8.
Quercetin, which is the most abundant bioflavonoid compound, is mainly present in the glycoside form of Quercitrin. Although different studies indicated that Quercitrin is a potent antioxidant, the action of this compound is not well understood. In this study, we investigated whether Quercitrin has apoptotic and antiproliferative effects in DLD-1 colon cancer cell lines. Time and dose dependent antiproliferative and apoptotic effects of Quercitrin were subsequently determined by WST-1 cell proliferation assay, lactate dehydrogenase (LDH) cytotoxicity assay, detection of nucleosome enrichment factor, changes in caspase-3 activity, loss of mitochondrial membrane potential (MMP) and also the localization of phosphatidylserine (PS) in the plasma membrane. There were significant increases in caspase-3 activity, loss of MMP, and increases in the apoptotic cell population in response to Quercitrin in DLD-1 colon cancer cells in a time- and dose-dependent manner. These results revealed that Quercitrin has antiproliferative and apoptotic effects on colon cancer cells. Quercitrin activity supported with in vivo analyses could be a biomarker candicate for early colorectal carcinoma.
Molecular mechanisms of quercitrin-induced apoptosis in non-small cell lung cancer.[Pubmed:25193878]
Arch Med Res. 2014 Aug;45(6):445-54.
BACKGROUND AND AIMS: Quercitrin (QR; quercetin-3-O-rhamnoside) has been used previously as an antibacterial agent and has been shown to inhibit the oxidation of low-density lipoproteins and prevent an allergic reaction. Furthermore, it was demonstrated that Quercitrin exerts protective effects against H2O2-induced dysfunction in lung fibroblast cells. However, the mechanisms of Quercitrin effects on cancer cell proliferation and apoptosis is not well understood. The aim of this study is to investigate the cytotoxic and apoptotic effects of Quercitrin and the molecular mechanisms of Quercitrin-induced apoptosis in non-small cell lung cancer (NSCLC) cell lines. METHODS: Time- and dose-dependent antiproliferative and apoptotic effects of Quercitrin determined by WST-1 cell proliferation assay, lactate dehydrogenase (LDH) cytotoxicity assay, determination of nucleosome enrichment factor, changes in caspase-3 activity, loss of mitochondrial membrane potential (MMP) and also the localization of phosphatidylserine in the plasma membrane. Changes in whole genome gene expression levels were examined by Illumina Human HT-12v4 beadchip microarrays. RESULTS: There were significant increases in caspase-3 activity, loss of MMP, and increases in apoptotic cell population in response to Quercitrin in A549 and NCI-H358 NSCLC cells in a time- and dose-dependent manner. CONCLUSION: Our results demonstrated that genes involved in leukocyte transendothelial migration, cell adhesion and phosphatidylinositol signaling system pathways were the most statistically significant pathways in NCI-H358 and A549 cells. These results revealed that Quercitrin has antiproliferative and apoptotic effects on lung cancer cells by modulating the immune response. After confirming its anticarcinogenic effects in vivo, Quercitrin could be a novel and strong anticancer agent against NSCLC.
In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway.[Pubmed:15668926]
Eur J Immunol. 2005 Feb;35(2):584-92.
Quercetin is a common antioxidant flavonoid found in vegetables, which is usually present in glycosylated forms, such as Quercitrin (3-rhamnosylquercetin). Previous in vitro experiments have shown that quercetin exerts a bigger effect than Quercitrin in the down-regulation of the inflammatory response. However, such results have not been reproduced in in vivo experimental models of intestinal inflammation, in which quercetin did not show beneficial effects while its glycosides, Quercitrin or rutin, have demonstrated their effectiveness. In this study, we have reported that the in vivo effects of Quercitrin in the experimental model of rat colitis induced by dextran sulfate sodium can be mediated by the release of quercetin generated after glycoside's cleavage by the intestinal microbiota. This is supported by the fact that quercetin, but not Quercitrin, is able to down-regulate the inflammatory response of bone marrow-derived macrophages in vitro. Moreover, we have demonstrated that quercetin inhibits cytokine and inducible nitric oxide synthase expression through inhibition of the NF-kappaB pathway without modification of c-Jun N-terminal kinase activity (both in vitro and in vivo). As a conclusion, our report suggests that Quercitrin releases quercetin in order to perform its anti-inflammatory effect which is mediated through the inhibition of the NF-kappaB pathway.
Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages.[Pubmed:15876423]
Biochem Pharmacol. 2005 Jun 15;69(12):1839-51.
In the present study, we examine the protective mechanism of quercetin (QE) on oxidative stress-induced cytotoxic effect in RAW264.7 macrophages. Results of Western blotting show that QE but not its glycoside rutin (RUT) and quicitrin-induced HO-1 protein expression in a time- and dose-dependent manner, and HO-1 protein induced by QE was blocked by an addition of cycloheximide or actinomycin D. Induction of HO-1 gene expression by QE was accompanied by inducing ERKs, but not JNKs or p38, proteins phosphorylation. Addition of PD98059, but not SB203580 or SP600125, significantly attenuates QE-induced HO-1 protein and mRNA expression associated with blocking the expression of phosphorylated ERKs proteins. H(2)O(2) addition reduces the viability of cells by MTT assay, and appearance of DNA ladders, hypodiploid cells, and an increase in intracellular peroxide level was detected. Addition of QE, but not QI or RUT, significantly reduced the cytotoxic effect induced by H(2)O(2) associated with blocking the production of intracellular peroxide, DNA ladders, and hypodiploid cells. QE protection of cells from H(2)O(2)-induced apoptosis was significantly suppressed by adding HO inhibitor SnPP or ERKs inhibitor PD98059. Additionally, QE protects cells from H(2)O(2)-induced a decrease in the mitochondrial membrane potential and a release of cytochrome c from mitochondria to cytosol by DiOC6 and Western blotting assay, respectively. Activation of apoptotic proteins including the caspase 3, caspase 9, PARP, D4-GDI proteins was identified in H(2)O(2)-treated cells by Western blotting and enzyme activity assay, and that was significantly blocked by an addition of QE, but not RUT and QI. Furthermore, HO-1 catalytic metabolites carbon monoxide (CO), but not Fe(2+), Fe(3+), biliverdin or bilirubin, performed protective effect on cells from H(2)O(2)-induced cell death with an increase in HO-1 protein expression and ERKs protein phosphorylation. These data suggest that induction of HO-1 protein may participate in the protective mechanism of QE on oxidative stress (H(2)O(2))-induced apoptosis, and reduction of intracellular ROS production and mitochondria dysfunction with blocking apoptotic events were involved. Differential anti-apoptotic effect between QE and its glycosides RUT and QI via distinct HO-1 protein induction was also delineated.
Inhibition of major virulence pathways of Streptococcus mutans by quercitrin and deoxynojirimycin: a synergistic approach of infection control.[Pubmed:24622055]
PLoS One. 2014 Mar 12;9(3):e91736.
OBJECTIVES: To evaluate the synergistic effect of Quercitrin and Deoxynojirimycin (DNJ) together with their individual inhibitory effect against virulence pathways of Streptococcus mutans. METHODOLOGY: MICs of both the compounds were determined by the microdilution method, followed by their in vitrosynergy using checkerboard and time kill assay. The nature of interaction was classified as synergistic on the basis of fractional inhibitory concentration index (FICI) value of Quercitrin and DNJ was evaluated individually and in combination against various cariogenic properties of S. mutans UA159 such as acidogenesis, aciduracity, glucan production, hydrophobicity, biofilm and adherence. Moreover, expression of virulent genes in S. mutans was analysed by quantitative RT- PCR (qRT-PCR) and inhibition of F1F0-ATPase, lactate dehydrogenase and enolase was also evaluated. Finally, scanning electron microscopy (SEM) was used to investigate structural obliteration of biofilm. RESULTS: The in vitro synergism between Quercitrin and DNJ was observed, with a FICI of 0.313. Their MIC values were found to be 64 mug/ml and 16 mug/ml respectively. The synergistic combination consistently showed best activity against all the virulence factors as compared to Quercitrin and DNJ individually. A reduction in glucan synthesis and biofilm formation was observed at different phases of growth. The qRT-PCR revealed significant downregulation of various virulent genes. Electron micrographs depicted the obliteration of biofilm as compared to control and the activity of cariogenic enzymes was also inhibited. CONCLUSIONS: The whole study reflects a prospective role of Quercitrin and DNJ in combination as a potent anticariogenic agent against S. mutans.


