KaranjinCAS# 521-88-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 521-88-0 | SDF | Download SDF |
| PubChem ID | 100633 | Appearance | White powder |
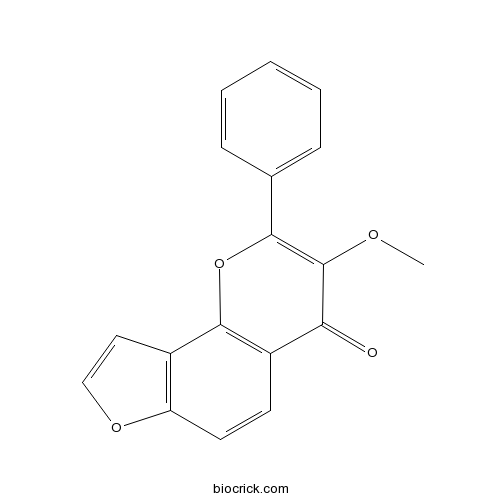
| Formula | C18H12O4 | M.Wt | 292.29 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform; sparingly soluble in water | ||
| Chemical Name | 3-methoxy-2-phenylfuro[2,3-h]chromen-4-one | ||
| SMILES | COC1=C(OC2=C(C1=O)C=CC3=C2C=CO3)C4=CC=CC=C4 | ||
| Standard InChIKey | LKPQNZRGGNOPPU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H12O4/c1-20-18-15(19)13-7-8-14-12(9-10-21-14)17(13)22-16(18)11-5-3-2-4-6-11/h2-10H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Karanjin possesses gastroprotective property. 2. Karanjin possesses significant antioxidant activity. 3. Karanjin has larvicidal activity toward C. pipiens pallens larvae and A. aegypti larvae. 4. Karanjin plays anti-inflammatory role in experimental arthritis model as well as on macrophage signalling. 5. Karanjin is a potent Volume-regulated anion channels(VRACs) current inhibitor, the VRAC inhibition might be responsible for its anti-angiogenic effects. 6. Karanjin has the potential to cure colitis induced by intracolonic administration of 2,4,6-trinitrobenzenesulfonic acid (TNBS). 7. Karanjin can significantly reverse the amnesia induced by diazepam and improve learning and memory of mice in dose and time dependent manner. 8. Karanjin can induce cancer cell death through cell cycle arrest and enhance apoptosis, it may be effective clinically for cancer pharmacotherapy. 9. Karanjin possesses significant antihyperglycemic activity in Streptozotocin-induced diabetic rats and type 2 diabetic db/db mice and protein tyrosine phosphatase-1B may be the possible target for their activity. |
| Targets | SOD | NO | AChR | TNF-α | NF-kB | GLUT | AMPK | PI3K | Akt | Sodium Channel | ATPase |

Karanjin Dilution Calculator

Karanjin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4213 mL | 17.1063 mL | 34.2126 mL | 68.4252 mL | 85.5315 mL |
| 5 mM | 0.6843 mL | 3.4213 mL | 6.8425 mL | 13.685 mL | 17.1063 mL |
| 10 mM | 0.3421 mL | 1.7106 mL | 3.4213 mL | 6.8425 mL | 8.5531 mL |
| 50 mM | 0.0684 mL | 0.3421 mL | 0.6843 mL | 1.3685 mL | 1.7106 mL |
| 100 mM | 0.0342 mL | 0.1711 mL | 0.3421 mL | 0.6843 mL | 0.8553 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Broxyquinoline
Catalog No.:BCC4642
CAS No.:521-74-4
- Cinnamoylcocaine
Catalog No.:BCN1429
CAS No.:521-67-5
- Frangulin A
Catalog No.:BCC8174
CAS No.:521-62-0
- Physcion
Catalog No.:BCN5663
CAS No.:521-61-9
- Vulpic acid
Catalog No.:BCN6546
CAS No.:521-52-8
- Pedicin
Catalog No.:BCN4845
CAS No.:521-51-7
- Cannabinol
Catalog No.:BCN7968
CAS No.:521-35-7
- Sciadopitysin
Catalog No.:BCN5662
CAS No.:521-34-6
- Bilobetin
Catalog No.:BCN5661
CAS No.:521-32-4
- Stanolone
Catalog No.:BCC9153
CAS No.:521-18-6
- Androstenediol
Catalog No.:BCC8828
CAS No.:521-17-5
- Dromostanolone propionate
Catalog No.:BCC8954
CAS No.:521-12-0
- 2,4-Dihydroxy-6-methoxy-3-formylacetophenone
Catalog No.:BCN1430
CAS No.:52117-67-6
- 3-O-Acetylpinobanksin
Catalog No.:BCN5660
CAS No.:52117-69-8
- H-Tyr(Bzl)-OBzl.HCl
Catalog No.:BCC3131
CAS No.:52142-01-5
- Piperitol
Catalog No.:BCN3968
CAS No.:52151-92-5
- 7-Hydroxy-2,3,4,5-tetrahydro-1H-benzofuro[2,3-c]azepin-1-one
Catalog No.:BCC3960
CAS No.:521937-07-5
- N'-Methylammodendrine
Catalog No.:BCN2147
CAS No.:52196-10-8
- Evoxine
Catalog No.:BCN5664
CAS No.:522-11-2
- Quercitrin
Catalog No.:BCN5665
CAS No.:522-12-3
- Deguelin
Catalog No.:BCN4804
CAS No.:522-17-8
- Norsanguinarine
Catalog No.:BCN3714
CAS No.:522-30-5
- Lochnerine
Catalog No.:BCN5667
CAS No.:522-47-4
- Tetrahydrozoline HCl
Catalog No.:BCC4339
CAS No.:522-48-5
Effects of karanjin on cell cycle arrest and apoptosis in human A549, HepG2 and HL-60 cancer cells.[Pubmed:26209237]
Biol Res. 2015 Jul 26;48:40.
BACKGROUND: We have investigated the potential anticancer effects of Karanjin, a principal furanoflavonol constituent of the Chinese medicine Fordia cauliflora, using cytotoxic assay, cell cycle arrest, and induction of apoptosis in three human cancer cell lines (A549, HepG2 and HL-60 cells). RESULTS: MTT cytotoxic assay showed that Karanjin could inhibit the proliferation and viability of all three cancer cells. The induction of cell cycle arrest was observed via a PI (propidium iodide)/RNase Staining Buffer detection kit and analyzed by flow cytometry: Karanjin could dose-dependently induce cell cycle arrest at G2/M phase in the three cell lines. Cell apoptosis was assessed by Annexin V-FITC/PI staining: all three cancer cells treated with Karanjin exhibited significantly increased apoptotic rates, especially in the percentage of late apoptosis cells. CONCLUSION: Karanjin can induce cancer cell death through cell cycle arrest and enhance apoptosis. This compound may be effective clinically for cancer pharmacotherapy.
Effect of karanjin on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in Balb/c mice.[Pubmed:28706329]
Indian J Pharmacol. 2017 Mar-Apr;49(2):161-167.
OBJECTIVES: The objective of this study is to evaluate the beneficial effect of Karanjin for the treatment of experimental colitis. METHODS: Colitis was induced in the Balb/c mice by rectal administration of 2% solution of 2,4,6-trinitrobenzenesulfonic acid (TNBS) in 50% methanol. Karanjin (>98% pure) was administered in two different concentrations 100 and 200 mg/kg and sulfasalazine (100 mg/kg) as reference for 7 consecutive days to colitic mice. On the 8 day, mice were euthanized and degree of inflammation was assessed by macroscopic, microscopic, histology and biochemical estimation of myeloperoxidase (MPO), nitric oxide (NO), malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), and reduced glutathione (GSH) level were measured. RESULTS: Karanjin significantly and dose dependently ameliorate the macroscopic damage, histological changes such as cellular infiltration, tissue necrosis, mucosal and submucosal damage as compared to the TNBS control group. Karanjin reduces the activity of MPO, depressed MDA, and NO level and helps in restoring the level of CAT, SOD, and GSH to normal when compared to the TNBS colitis group. CONCLUSION: Result of the present study indicates that Karanjin has the potential to cure colitis induced by intracolonic administration of TNBS.
Karanjin interferes with ABCB1, ABCC1, and ABCG2.[Pubmed:24735762]
J Pharm Pharm Sci. 2014;17(1):92-105.
PURPOSE: The prominent ATP-binding cassette (ABC) transporters ABCB1, ABCC1, and ABCG2 are involved in substance transport across physiological barriers and therefore in drug absorption, distribution, and elimination. They also mediate multi-drug resistance in cancer cells. Different flavonoids are known to interfere with different ABC transporters. Here, the effect of the furanoflavonol Karanjin, a potential drug with antiglycaemic, gastroprotective, antifungal, and antibacterial effects, was investigated on ABCB1, ABCC1, and ABCG2-mediated drug transport in comparison to the flavonoids apigenin, genistein, and naringenin. METHODS: Cells expressing the relevant transporters (ABCB1: UKF-NB-3(ABCB1), UKF-NB-3(r)VCR(1)(0); ABCC1: G62, PC-3(r)VCR(2)(0); ABCG2: UKF-NB-3(ABCG2)) were used in combination with specific fluorescent and cytotoxic ABC transporter substrates and ABC transporter inhibitors to study ABC transporter function. Moreover, the effects of the investigated flavonoids were determined on the ABC transporter ATPase activities. RESULTS: Karanjin interfered with drug efflux mediated by ABCB1, ABCC1, and ABCG2 and enhanced the ATPase activity of all three transporters. Moreover, Karanjin exerted more pronounced effects than the control flavonoids apigenin, genistein, and naringenin on all three transporters. Most notably, Karanjin interfered with ABCB1 at low concentrations being about 1 microM. CONCLUSIONS: Taken together, these findings should be taken into account during further consideration of Karanjin as a potential drug for different therapeutic indications. The effects on ABCB1, ABCC1, and ABCG2 may affect the pharmacokinetics of co-administered drugs.
Flavonoids from twigs of Millettia pubinervis.[Pubmed:25632467]
Nat Prod Commun. 2014 Dec;9(12):1721-2.
A new flavone, 3-methoxy-5-hydroxy-[2",3":7,8] furanoflavone, pubinerone (1), was isolated from the twigs of Millettia pubinervis Kurz, together with ten known flavonoids, Karanjin (2), kanjone (3), 3,6-dimethoxy-[2",3":7,8] furanoflavone (4), pongaglabrone (5), pongapin (6), pongaflavone (7), 3,6-dimethoxy- 6",6"-dimethylchromene-[2",3":7,8] flavone (8), pongachromene (9), 3,6-dimethoxy-3',4'-methylenedioxy-6",6"-dimethylchromene-[2",3":7,8] flavone (10) and demethoxykanugin (11). This is the first phytochemical investigation of this plant. The structure of compound 1 was elucidated on the basis of spectroscopic data interpretation, including 1D and 2D NMR and HREIMS analysis. The cytotoxicity of 1 against five human cancer cell lines; HL-60, SMMC-7721, A-549, MCF-7 and SW480, was evaluated, but it was inactive (IC50 > 40 muM).
Development and validation of an LC-MS method for determination of Karanjin in rat plasma: application to preclinical pharmacokinetics.[Pubmed:25002684]
J Chromatogr Sci. 2015 Apr;53(4):456-61.
A selective and sensitive liquid chromatography-mass spectrometry (MS) method was developed and validated for the determination of Karanjin in rat plasma. The target analyte, together with the internal standard (warfarin), was extracted from rat plasma by liquid-liquid extraction with ethyl acetate. Chromatographic separation was performed on a ZORBAX SB-C18 column using a mixture of acetonitrile and 0.1% aqueous formic acid as the mobile phase with linear gradient elution. MS detection was performed on a single quadrupole MS by selected ion monitoring mode via a positive electrospray ionization source. The assay exhibited a linear dynamic range of 2.50-3,000 ng/mL for Karanjin. The intra- and inter-day precision was <10.8%, and the intra- and inter-day accuracy was <9.2%. The validated method has been applied to the preclinical pharmacokinetic studies of Karanjin following oral administration of 5, 10 and 20 mg/kg Karanjin to rats.
A new 1,2-ethanedione benzofurane derivative from Tephrosia purpurea.[Pubmed:25116833]
Nat Prod Res. 2014;28(20):1705-8.
A new 1,2-ethanedione benzofurane derivative, purpdione (1), was isolated from Tephrosia purpurea, together with seven known flavonoids, purpurenone (2), pongamol (3), ovalitenin A (4), Karanjin (5), lanceolatin B (6), tachrosin (7) and villosinol (8). The new structure was elucidated based on the analysis of its spectroscopic data. The structures of the known compounds were identified by comparing their spectroscopic data with those reported in the literature. The isolates exhibited marginal ability to inhibit the settlement of barnacle (Balanus reticulatus).
Comparative toxicity of an acetogenin-based extract and commercial pesticides against citrus red mite.[Pubmed:24696362]
Exp Appl Acarol. 2014;64(1):87-98.
Acetogenins, a class of natural compounds produced by some Annonaceae species, are potent inhibitors of mitochondrial electron transport systems. Although the cellular respiration processes are an important biochemical site for the acaricidal action of compounds, few studies have been performed to assess the bioactivity of acetogenin-based biopesticides on spider mites, mainly against species that occur in orchards. Using residual contact bioassays, this study aimed to evaluate the bioactivity of an ethanolic extract from Annona mucosa seeds (ESAM) (Annonaceae) against the citrus red mite Panonychus citri (McGregor) (Acari: Tetranychidae), an important pest of the Brazilian citriculture. ESAM is a homemade biopesticide which was previously characterized by its high concentration of acetogenins. It caused both high mortality of P. citri females (LC50 = 7,295, 4,662, 3,463, and 2,608 mg l(-1), after 48, 72, 96, and 120 h of exposure, respectively) and significant oviposition deterrence (EC50 = 3.194,80 mg l(-1)). However, there was no effect on P. citri female fertility (hatching rate). In addition, the ESAM efficacy (in terms of its LC90) was compared with commercial acaricides/insecticides (at its recommended rate) of both natural [Anosom((R)) 1 EC (annonin), Derisom((R)) 2 EC (Karanjin), and Azamax((R)) 1.2 EC (azadirachtin + 3-tigloylazadirachtol)] and synthetic origin [Envidor((R)) 24 SC (spirodiclofen)]. Based on all of the analyzed variables, the ESAM exhibited levels of activity superior to other botanical commercial acaricides and similar to spirodiclofen. Thus, our results indicate that ESAM may constitute a biorational acaricide for citrus red mite integrated pest management in Brazilian citrus orchards, particularly for local use.


