GSK1324726ABET proteins inhibitor CAS# 1300031-52-0 |
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1300031-52-0 | SDF | Download SDF |
| PubChem ID | 52912222 | Appearance | Powder |
| Formula | C25H23ClN2O3 | M.Wt | 434.91 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 46 mg/mL (105.77 mM) *"≥" means soluble, but saturation unknown. | ||
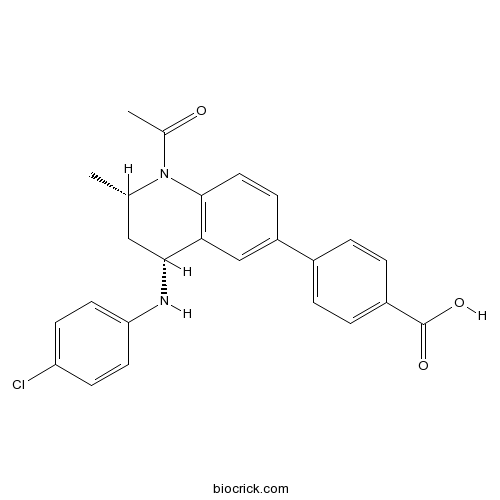
| Chemical Name | 4-[(2S,4R)-1-acetyl-4-(4-chloroanilino)-2-methyl-3,4-dihydro-2H-quinolin-6-yl]benzoic acid | ||
| SMILES | CC1CC(C2=C(N1C(=O)C)C=CC(=C2)C3=CC=C(C=C3)C(=O)O)NC4=CC=C(C=C4)Cl | ||
| Standard InChIKey | FAWSUKOIROHXAP-NPMXOYFQSA-N | ||
| Standard InChI | InChI=1S/C25H23ClN2O3/c1-15-13-23(27-21-10-8-20(26)9-11-21)22-14-19(7-12-24(22)28(15)16(2)29)17-3-5-18(6-4-17)25(30)31/h3-12,14-15,23,27H,13H2,1-2H3,(H,30,31)/t15-,23+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GSK1324726A is a selective small-molecule inhibitor of BET proteins with IC50 values of 41 nM, 31 nM and 22 nM for BRD2, BRD3 and BRD4, respectively . | |||||
| Targets | BRD2 | BRD3 | BRD4 | |||
| IC50 | 41 nM | 31 nM | 22 nM | |||

GSK1324726A Dilution Calculator

GSK1324726A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2993 mL | 11.4966 mL | 22.9933 mL | 45.9865 mL | 57.4832 mL |
| 5 mM | 0.4599 mL | 2.2993 mL | 4.5987 mL | 9.1973 mL | 11.4966 mL |
| 10 mM | 0.2299 mL | 1.1497 mL | 2.2993 mL | 4.5987 mL | 5.7483 mL |
| 50 mM | 0.046 mL | 0.2299 mL | 0.4599 mL | 0.9197 mL | 1.1497 mL |
| 100 mM | 0.023 mL | 0.115 mL | 0.2299 mL | 0.4599 mL | 0.5748 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GSK1324726A is a selective small molecule inhibitor of BET proteins with IC50 values of 41nM, 31nM and 22nM, respectively for BRD2, BRD3 and BRD4 [1].
GSK1324726A competes with histone H4 peptides for binding to the bromodomains of the BET proteins. It notably inhibits cell growth in neuroblastoma cell lines with a median GI50 value of 75nM. And it is also found to cause some level of net cell death. The mechanism of this is that GSK1324726A induces cytotoxicity in these cell lines. Treatment of GSK1324726A results in an induction of G1 arrest in SK–N-AS, SK–N–SH and CHP-212 cell lines. Meanwhile, caspase 3/7 induction is observed in SK–N–SH and CHP-212 cell lines. In mice bearing the neuroblastoma xenografts, GSK1324726A also shows the potency of inhibiting tumor growth [1].
References:
[1] Wyce A, Ganji G, Smitheman KN, Chung CW, Korenchuk S, Bai Y, Barbash O, Le B, Craggs PD, McCabe MT, Kennedy-Wilson KM, Sanchez LV, Gosmini RL, Parr N, McHugh CF, Dhanak D, Prinjha RK, Auger KR, Tummino PJ. BET inhibition silences expression of MYCN and BCL2 and induces cytotoxicity in neuroblastoma tumor models. PLoS One. 2013 Aug 23;8(8):e72967.
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- Quinine
Catalog No.:BCN2341
CAS No.:130-95-0
- Quinine HCl
Catalog No.:BCN2262
CAS No.:130-89-2
- Protopine
Catalog No.:BCN6165
CAS No.:130-86-9
- Thioridazine HCl
Catalog No.:BCC3869
CAS No.:130-61-0
- 1,4-Naphthoquinone
Catalog No.:BCN8420
CAS No.:130-15-4
- Senecionine
Catalog No.:BCN2129
CAS No.:130-01-8
- Iloperidone hydrochloride
Catalog No.:BCC4212
CAS No.:1299470-39-5
- Dapoxetine HCl
Catalog No.:BCC5064
CAS No.:129938-20-1
- Amicarbazone
Catalog No.:BCC5464
CAS No.:129909-90-6
- MC 976
Catalog No.:BCC1734
CAS No.:129831-99-8
- ODM-201
Catalog No.:BCC3796
CAS No.:1297538-32-9
- Dehydrocorydaline nitrate
Catalog No.:BCN2745
CAS No.:13005-09-9
- Mafenide Acetate
Catalog No.:BCC5236
CAS No.:13009-99-9
- Lomustine
Catalog No.:BCC4794
CAS No.:13010-47-4
- (+)-Igmesine hydrochloride
Catalog No.:BCC5902
CAS No.:130152-35-1
- Ergosterol glucoside
Catalog No.:BCN7327
CAS No.:130155-33-8
- Torilolone
Catalog No.:BCN6659
CAS No.:13018-09-2
- Torilin
Catalog No.:BCN6611
CAS No.:13018-10-5
- Cirsimarin
Catalog No.:BCN6821
CAS No.:13020-19-4
- N-(4-Hydroxyphenylacetyl)spermine
Catalog No.:BCC6594
CAS No.:130210-32-1
- Acerogenin G
Catalog No.:BCN7328
CAS No.:130233-83-9
- 3'-Demethoxypiplartine
Catalog No.:BCN4021
CAS No.:130263-10-4
- PD 135158
Catalog No.:BCC7431
CAS No.:130285-87-9
The discovery of I-BET726 (GSK1324726A), a potent tetrahydroquinoline ApoA1 up-regulator and selective BET bromodomain inhibitor.[Pubmed:25249180]
J Med Chem. 2014 Oct 9;57(19):8111-31.
Through their function as epigenetic readers of the histone code, the BET family of bromodomain-containing proteins regulate expression of multiple genes of therapeutic relevance, including those involved in tumor cell growth and inflammation. BET bromodomain inhibitors have profound antiproliferative and anti-inflammatory effects which translate into efficacy in oncology and inflammation models, and the first compounds have now progressed into clinical trials. The exciting biology of the BETs has led to great interest in the discovery of novel inhibitor classes. Here we describe the identification of a novel tetrahydroquinoline series through up-regulation of apolipoprotein A1 and the optimization into potent compounds active in murine models of septic shock and neuroblastoma. At the molecular level, these effects are produced by inhibition of BET bromodomains. X-ray crystallography reveals the interactions explaining the structure-activity relationships of binding. The resulting lead molecule, I-BET726, represents a new, potent, and selective class of tetrahydroquinoline-based BET inhibitors.


