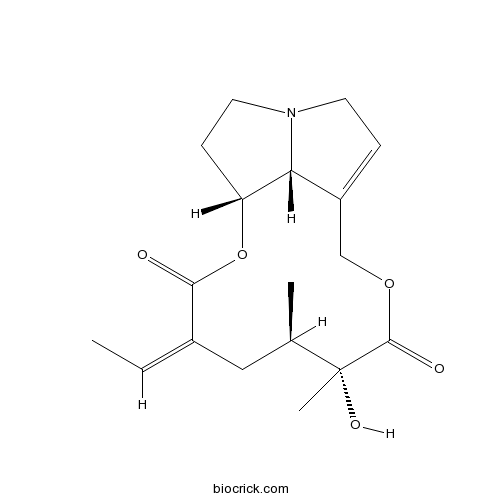
SenecionineCAS# 130-01-8 |
- Integerrimine
Catalog No.:BCN2131
CAS No.:480-79-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 130-01-8 | SDF | Download SDF |
| PubChem ID | 5280906 | Appearance | White powder |
| Formula | C18H25NO5 | M.Wt | 335.40 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Aureine | ||
| Solubility | Soluble in chloroform | ||
| SMILES | CC=C1CC(C(C(=O)OCC2=CCN3C2C(CC3)OC1=O)(C)O)C | ||
| Standard InChIKey | HKODIGSRFALUTA-JTLQZVBZSA-N | ||
| Standard InChI | InChI=1S/C18H25NO5/c1-4-12-9-11(2)18(3,22)17(21)23-10-13-5-7-19-8-6-14(15(13)19)24-16(12)20/h4-5,11,14-15,22H,6-10H2,1-3H3/b12-4-/t11-,14-,15-,18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Senecionine (SEN) is a representative of the hepatotoxic pyrrolizidine alkaloids. |
| Targets | P450 (e.g. CYP17) | Calcium Channel |

Senecionine Dilution Calculator

Senecionine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9815 mL | 14.9076 mL | 29.8151 mL | 59.6303 mL | 74.5379 mL |
| 5 mM | 0.5963 mL | 2.9815 mL | 5.963 mL | 11.9261 mL | 14.9076 mL |
| 10 mM | 0.2982 mL | 1.4908 mL | 2.9815 mL | 5.963 mL | 7.4538 mL |
| 50 mM | 0.0596 mL | 0.2982 mL | 0.5963 mL | 1.1926 mL | 1.4908 mL |
| 100 mM | 0.0298 mL | 0.1491 mL | 0.2982 mL | 0.5963 mL | 0.7454 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Iloperidone hydrochloride
Catalog No.:BCC4212
CAS No.:1299470-39-5
- Dapoxetine HCl
Catalog No.:BCC5064
CAS No.:129938-20-1
- Amicarbazone
Catalog No.:BCC5464
CAS No.:129909-90-6
- MC 976
Catalog No.:BCC1734
CAS No.:129831-99-8
- ODM-201
Catalog No.:BCC3796
CAS No.:1297538-32-9
- Anemoside B4
Catalog No.:BCN1276
CAS No.:129741-57-7
- Anemoside A3
Catalog No.:BCN2328
CAS No.:129724-84-1
- 3-Hydroxylanost-9(11)-24-dien-26-oic acid
Catalog No.:BCN1586
CAS No.:129724-83-0
- 2',4'-Dihydroxy-3',6'-dimethoxychalcone
Catalog No.:BCN6158
CAS No.:129724-43-2
- Aripiprazole
Catalog No.:BCC5034
CAS No.:129722-12-9
- Dofequidar
Catalog No.:BCC4176
CAS No.:129716-58-1
- ZD 7114 hydrochloride
Catalog No.:BCC6852
CAS No.:129689-28-7
- 1,4-Naphthoquinone
Catalog No.:BCN8420
CAS No.:130-15-4
- Thioridazine HCl
Catalog No.:BCC3869
CAS No.:130-61-0
- Protopine
Catalog No.:BCN6165
CAS No.:130-86-9
- Quinine HCl
Catalog No.:BCN2262
CAS No.:130-89-2
- Quinine
Catalog No.:BCN2341
CAS No.:130-95-0
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- Dehydrocorydaline nitrate
Catalog No.:BCN2745
CAS No.:13005-09-9
- Mafenide Acetate
Catalog No.:BCC5236
CAS No.:13009-99-9
- Lomustine
Catalog No.:BCC4794
CAS No.:13010-47-4
- (+)-Igmesine hydrochloride
Catalog No.:BCC5902
CAS No.:130152-35-1
- Ergosterol glucoside
Catalog No.:BCN7327
CAS No.:130155-33-8
Identification of the UDP-glucuronosyltransferase isozyme involved in senecionine glucuronidation in human liver microsomes.[Pubmed:20056725]
Drug Metab Dispos. 2010 Apr;38(4):626-34.
Senecionine (SEN) is a representative of the hepatotoxic pyrrolizidine alkaloids. Although phase I metabolism for cytochrome P450-mediated metabolic activation of SEN was investigated extensively, phase II metabolism for glucuronidation of this compound has not been investigated until now. In our present study, one unique glucuronidation product of SEN in human liver microsomes (HLMs) was identified as SEN N-glucuronide using an authentically synthesized product for which the structure was identified via (1)H and (13)C NMR analysis. Subsequently, kinetics indicated that SEN N-glucuronidation followed the typical Michaelis-Menten model and only one major isozyme participated in it. Finally, this isozyme was demonstrated to be UDP-glucuronosyltransferase (UGT) 1A4, with the direct evidence that recombinant UGT1A4 exhibited predominant and exclusive activity on SEN N-glucuronidation. This result was confirmed by other experiments including chemical inhibition by selective inhibitors and a correlation study between activities of SEN N-glucuronidation and various UGT isozymes. The exclusive role of UGT1A4 on SEN N-glucuronidation was strengthened additionally by its inhibitory kinetic study in which the selective inhibitor of UGT1A4 showed a similar inhibition pattern and K(i) values in both HLM and recombinant UGT1A4 systems. Because UGT2B10 activity failed to correlate with SEN N-glucuronidation in HLMs from 10 individuals, it was impossible for UGT2B10 to play an important role in this metabolism.
Effects of the pyrrolizidine alkaloid senecionine and the alkenals trans-4-OH-hexenal and trans-2-hexenal on intracellular calcium compartmentation in isolated hepatocytes.[Pubmed:2492804]
Biochem Pharmacol. 1989 Feb 1;38(3):391-7.
The pyrrolizidine alkaloid Senecionine has been shown to produce an increase in cytosolic free Ca2+ concentration in isolated hepatocytes that correlated with an increase in cellular toxicity. The cytotoxicity was greater in the absence of extracellular Ca2+ than in its presence, suggesting that alterations in intracellular Ca2+ distribution, and not an influx of extracellular Ca2+, were responsible for the Senecionine-induced hepatotoxicity. The effect of Senecionine, as well as the effects of trans-4-OH-2-hexenal (t-4HH), a microsomal metabolite of Senecionine, and a related alkenal, trans-2-hexenal, on the sequestration of Ca2+ in mitochondrial and extramitochondrial compartments were examined in isolated hepatocytes. Each of the test compounds elicited a decrease in the available extramitochondrial Ca2+ stores that was inhibited by pretreatment with the thiol group reducing agent, dithiothreitol. Senecionine and t-4HH decreased the level of Ca2+ sequestered in the mitochondrial compartment of hepatocytes. The presence of a pyridine nucleotide reducing agent, beta-hydroxybutyrate, inhibited this reduction. These results suggest that both Senecionine and t-4HH inhibit the sequestration of Ca2+ in extramitochondrial and mitochondrial compartments possibly by inactivating free sulfhydryl groups and oxidizing pyridine nucleotides respectively.
Metabolomic and genomic evidence for compromised bile acid homeostasis by senecionine, a hepatotoxic pyrrolizidine alkaloid.[Pubmed:24641316]
Chem Res Toxicol. 2014 May 19;27(5):775-86.
Pyrrolizidine alkaloids (PAs) are among the most hepatotoxic natural products that produce irreversible injury to humans via the consumption of herbal medicine and honey, and through tea preparation. Toxicity and death caused by PA exposure have been reported worldwide. Metabolomics and genomics provide scientific and systematic views of a living organism and have become powerful techniques for toxicology research. In this study, Senecionine hepatotoxicity on rats was determined via a combination of metabolomic and genomic analyses. From the global analysis generated from two omics data, the compromised bile acid homeostasis in vivo was innovatively demonstrated and confirmed. Serum profiling of bile acids was altered with significantly elevated conjugated bile acids after Senecionine exposure, which was in accordance with toxicity. Similarly, the hepatic mRNA levels of several key genes associated with bile acid metabolism were significantly changed. This process included cholesterol 7-alpha hydroxylase, bile acid CoA-amino acid N-acetyltransferase, sodium taurocholate cotransporting polypeptide, organic anion-transporting polypeptides, and multidrug-resistance-associated protein 3. In conclusion, a cross-omics study provides a comprehensive analysis method for studying the toxicity caused by Senecionine, which is a hepatotoxic PA. Moreover, the change in bile acid metabolism and the respective transporters may provide a new PA toxicity mechanism.
Role of cytochrome P450IIIA4 in the metabolism of the pyrrolizidine alkaloid senecionine in human liver.[Pubmed:2009596]
Carcinogenesis. 1991 Mar;12(3):515-9.
Studies were carried out to investigate the metabolism of Senecionine by human liver microsomes and the role of human cytochrome P450IIIA4 in this process. Human liver microsomes metabolized Senecionine to two major products, (+/-)-6,7-dihydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine (DHP) and Senecionine N-oxide. The rates of product formation (DHP and Senecionine N-oxide) varied widely with the microsomal samples tested. There was a 30-fold difference in DHP formation and a 25-fold difference in N-oxidation between the poorest metabolizer and the highest metabolizer of Senecionine. The conversion of Senecionine to DHP and Senecionine N-oxide in human liver microsomes was markedly inhibited by the mechanism-based inactivators of P450IIIA4, gestodene and triacetyloleandomycin. Anti-P450IIIA4 IgG, at a concentration of 1 mg/nmol of P450, was found to inhibit completely the formation of DHP and Senecionine N-oxide in human liver microsomes (HL101) having low activity toward Senecionine. At 5 mg IgG/nmol P450, anti-P450IIIA4 inhibited 90 and 84% respectively of the formation of DHP and Senecionine N-oxide in liver microsomes (HL110) with the highest activity toward Senecionine. The formation of DHP or Senecionine N-oxide was highly correlated with the amount of P450IIIA4 measured in the microsomes using polyclonal anti-P450IIIA4 IgG. The rate of DHP production also had a strong correlation with the rate of Senecionine N-oxide formation (r = 0.999) and with the rate of nifedipine oxidation (r = 0.998). Our present studies provide evidence that P450IIIA4 is the major enzyme catalyzing the bioactivation (DHP formation) and detoxication (Senecionine N-oxide formation) of Senecionine in human liver.


