AmicarbazonePET inhibitor CAS# 129909-90-6 |
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- p53 and MDM2 proteins-interaction-inhibitor racemic
Catalog No.:BCC1831
CAS No.:939983-14-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 129909-90-6 | SDF | Download SDF |
| PubChem ID | 153920 | Appearance | Powder |
| Formula | C10H19N5O2 | M.Wt | 241.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BAY314666; BAY-MKH 3586 | ||
| Solubility | H2O : 1 mg/mL (4.14 mM; ultrasonic and heat to 50°C) | ||
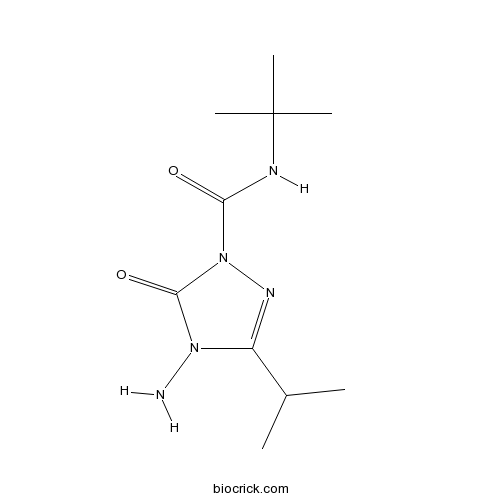
| Chemical Name | 4-amino-N-tert-butyl-5-oxo-3-propan-2-yl-1,2,4-triazole-1-carboxamide | ||
| SMILES | CC(C)C1=NN(C(=O)N1N)C(=O)NC(C)(C)C | ||
| Standard InChIKey | ORFPWVRKFLOQHK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H19N5O2/c1-6(2)7-13-15(9(17)14(7)11)8(16)12-10(3,4)5/h6H,11H2,1-5H3,(H,12,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Amicarbazone(BAY-MKH3586; BAY314666) is a potent inhibitor of photosynthetic electron transport via binding to the Qb domain of photosystem II (PSII); herbicide with a broad spectrum of weed control.
IC50 value:
Target: PSII inhibitor
The phenotypic responses of sensitive plants exposed to amicarbazone include chlorosis, stunted growth, tissue necrosis, and death. Its efficacy as both a foliar- and root-applied herbicide suggests that absorption and translocation of this compound is very rapid. As a result, its efficacy is susceptible to the most common form of resistance to PSII inhibitors. Nonetheless, amicarbazone has a good selectivity profile and is a more potent herbicide than atrazine, which enables its use at lower rates than those of traditional photosynthetic inhibitors. References: | |||||

Amicarbazone Dilution Calculator

Amicarbazone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1444 mL | 20.722 mL | 41.4439 mL | 82.8878 mL | 103.6098 mL |
| 5 mM | 0.8289 mL | 4.1444 mL | 8.2888 mL | 16.5776 mL | 20.722 mL |
| 10 mM | 0.4144 mL | 2.0722 mL | 4.1444 mL | 8.2888 mL | 10.361 mL |
| 50 mM | 0.0829 mL | 0.4144 mL | 0.8289 mL | 1.6578 mL | 2.0722 mL |
| 100 mM | 0.0414 mL | 0.2072 mL | 0.4144 mL | 0.8289 mL | 1.0361 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Amicarbazone(BAY-MKH3586; BAY314666) is a potent inhibitor of photosynthetic electron transport via binding to the Qb domain of photosystem II (PSII); herbicide with a broad spectrum of weed control.
- MC 976
Catalog No.:BCC1734
CAS No.:129831-99-8
- ODM-201
Catalog No.:BCC3796
CAS No.:1297538-32-9
- Anemoside B4
Catalog No.:BCN1276
CAS No.:129741-57-7
- Anemoside A3
Catalog No.:BCN2328
CAS No.:129724-84-1
- 3-Hydroxylanost-9(11)-24-dien-26-oic acid
Catalog No.:BCN1586
CAS No.:129724-83-0
- 2',4'-Dihydroxy-3',6'-dimethoxychalcone
Catalog No.:BCN6158
CAS No.:129724-43-2
- Aripiprazole
Catalog No.:BCC5034
CAS No.:129722-12-9
- Dofequidar
Catalog No.:BCC4176
CAS No.:129716-58-1
- ZD 7114 hydrochloride
Catalog No.:BCC6852
CAS No.:129689-28-7
- 3,4-Dihydroxybisabola-1,10-diene
Catalog No.:BCN7326
CAS No.:129673-87-6
- 3-Hydroxybisabola-1,10-dien-9-one
Catalog No.:BCN7325
CAS No.:129673-86-5
- GR 82334
Catalog No.:BCC5802
CAS No.:129623-01-4
- Dapoxetine HCl
Catalog No.:BCC5064
CAS No.:129938-20-1
- Iloperidone hydrochloride
Catalog No.:BCC4212
CAS No.:1299470-39-5
- Senecionine
Catalog No.:BCN2129
CAS No.:130-01-8
- 1,4-Naphthoquinone
Catalog No.:BCN8420
CAS No.:130-15-4
- Thioridazine HCl
Catalog No.:BCC3869
CAS No.:130-61-0
- Protopine
Catalog No.:BCN6165
CAS No.:130-86-9
- Quinine HCl
Catalog No.:BCN2262
CAS No.:130-89-2
- Quinine
Catalog No.:BCN2341
CAS No.:130-95-0
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- Dehydrocorydaline nitrate
Catalog No.:BCN2745
CAS No.:13005-09-9
- Mafenide Acetate
Catalog No.:BCC5236
CAS No.:13009-99-9
Amicarbazone.[Pubmed:23634130]
Acta Crystallogr Sect E Struct Rep Online. 2013 Mar 28;69(Pt 4):o603.
Three independent mol-ecules comprise the asymmetric unit of the title compound, C10H19N5O2, (systematic name: 4-amino-N-tert-butyl-3-isopropyl-5-oxo-4,5-dihydro-1H-1,2,4-triazole-1-carboxamid e) . In all three mol-ecules, the triazole ring and the carboxamide group are almost coplanar [within 4.0-5.9 (9) degrees ], particularly because of the formation of an intra-molecular N-Hcdots, three dots, centeredO hydrogen bond. On other hand, the orientation of the isopropyl group varies significantly from mol-ecule to mol-ecule. The crystal packing is dominated by N-Hcdots, three dots, centeredO and N-Hcdots, three dots, centeredN hydrogen bonds, which connect the mol-ecules into infinite chains along [010].
Physiological effects of temperature on turfgrass tolerance to amicarbazone.[Pubmed:25045054]
Pest Manag Sci. 2015 Apr;71(4):571-8.
BACKGROUND: Amicarbazone effectively controls annual bluegrass (Poa annua L.) in bermudagrass [Cynodon dactylon (L.) Pers. x C. transvaalensis Burtt-Davy] and tall fescue (Festuca arundinacea Schreb.) with spring applications, but summer applications may excessively injure tall fescue. The objective of this research was to investigate physiological effects of temperature on Amicarbazone efficacy, absorption, translocation and metabolism in annual bluegrass, bermudagrass and tall fescue. RESULTS: At 25/20 degrees C (day/night), annual bluegrass absorbed 58 and 40% more foliar-applied Amicarbazone than bermudagrass and tall fescue, respectively, after 72 h. Foliar absorption increased at 40/35 degrees C in all species, compared with 25/20 degrees C, and tall fescue had similar absorption to annual bluegrass at 40/35 degrees C. At 6 days after treatment, annual bluegrass metabolized 54% of foliar-applied Amicarbazone, while bermudagrass and tall fescue metabolized 67 and 64% respectively. CONCLUSION: Tall fescue is more tolerant to Amicarbazone than annual bluegrass at moderate temperatures ( approximately 25/20 degrees C) owing to less absorption and greater metabolism. However, tall fescue susceptibility to Amicarbazone injury at high temperatures (40/35 degrees C) results from enhanced herbicide absorption compared with lower temperatures (25/20 degrees C). Bermudagrass is more tolerant to Amicarbazone than annual bluegrass and tall fescue owing to less herbicide absorption, regardless of temperature.
Dissipation kinetics and degradation mechanism of amicarbazone in soil revealed by a reliable LC-MS/MS method.[Pubmed:26139399]
Environ Sci Pollut Res Int. 2015 Nov;22(22):17518-26.
A sensitive and reliable analytical method was developed for simultaneous determination of Amicarbazone (AMZ) and its two major metabolites including desamino Amicarbazone (DA) and isopropyl-2-hydroxy-DA-Amicarbazone (Ipr-2-OH-DA-AMZ) in soil for the first time. Targeted analytes were extracted and purified using a modified quick, easy, cheap, effective, rugged, and safe (QuEChERS) procedure, and then analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) with a total run time of 9 min. The established approach was extensively validated by determining the linearity (R (2) >/= 0.99), recovery (84-96 ), sensitivity (limits of quantification at 5-10 mug kg(-1)), and precision (RSDs Amicarbazone in soil were thoroughly investigated in an illumination incubator. As revealed, AMZ was easily degraded with the half-lives of 13.9-19.7 days in soil. Field trial results of AMZ (40 g a.i. ha(-1)) in Shanghai showed that the residues of AMZ and its metabolite Ipr-2-OH-DA-AMZ decreased from 0.505 mg kg(-1) (day 50) to 0.038 mg kg(-1) (day 365) and from 0.099 mg kg(-1) (day 50) to 0.028 mg kg(-1) (day 365), respectively, while the content of DA increased from 0.097 mg kg(-1) (day 50) to 0.245 mg kg(-1) (day 365). This study provided valuable data to understand the toxicity of AMZ and substantially promote its safe application to protect environment and human health.
Analysis of amicarbazone and its two metabolites in grains and soybeans by liquid chromatography with tandem mass spectrometry.[Pubmed:25907799]
J Sep Sci. 2015 Jul;38(13):2245-52.
A sensitive, simple and reliable analytical method based on a modified quick, easy, cheap, effective, rugged, safe sample preparation and liquid chromatography with tandem mass spectrometry detection was developed for the simultaneous determination of Amicarbazone and its two major metabolites desamino Amicarbazone and isopropyl-2-hydroxy-desamino Amicarbazone residues in grains (rice, wheat, corn, buckwheat) and soybean. Several parameters, including liquid chromatography and tandem mass spectrometry conditions, extraction approaches and the adsorbents for clean-up, which might influence the accuracy of the method, were extensively investigated. The established method was further validated by determining the linearity (R(2) > 0.99), fortified recovery (79-118%), precision (1-12%) and sensitivity (limit of quantification, 5 mug/kg for Amicarbazone and desamino Amicarbazone, and 10 mug/kg for isopropyl-2-hydroxy-desamino Amicarbazone). Finally, the established method was successfully applied to determine the residues of Amicarbazone and its metabolites in 49 real samples of grain and soybean.


