p53 and MDM2 proteins-interaction-inhibitor chiralP53 and MDM2 proteins-interaction-inhibitor CAS# 939981-37-0 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- CDK4 inhibitor
Catalog No.:BCC4242
CAS No.:1256963-02-6
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- PHA-848125
Catalog No.:BCC3839
CAS No.:802539-81-7
- PD 0332991 (Palbociclib) HCl
Catalog No.:BCC3680
CAS No.:827022-32-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 939981-37-0 | SDF | Download SDF |
| PubChem ID | 17754765 | Appearance | Powder |
| Formula | C40H49Cl2N5O4 | M.Wt | 734.75 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (68.05 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
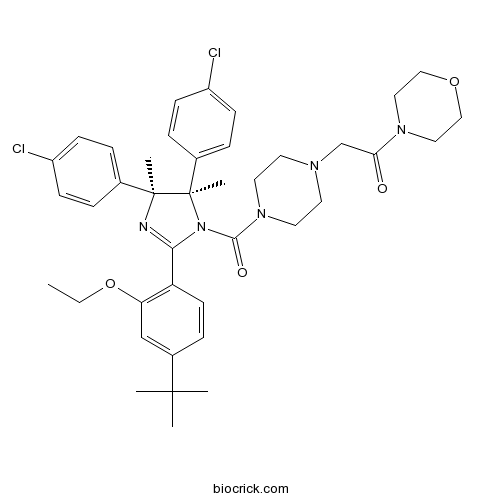
| Chemical Name | 2-[4-[(4S,5R)-2-(4-tert-butyl-2-ethoxyphenyl)-4,5-bis(4-chlorophenyl)-4,5-dimethylimidazole-1-carbonyl]piperazin-1-yl]-1-morpholin-4-ylethanone | ||
| SMILES | CCOC1=C(C=CC(=C1)C(C)(C)C)C2=NC(C(N2C(=O)N3CCN(CC3)CC(=O)N4CCOCC4)(C)C5=CC=C(C=C5)Cl)(C)C6=CC=C(C=C6)Cl | ||
| Standard InChIKey | DJZBZZRLUQDRII-IOLBBIBUSA-N | ||
| Standard InChI | InChI=1S/C40H49Cl2N5O4/c1-7-51-34-26-30(38(2,3)4)12-17-33(34)36-43-39(5,28-8-13-31(41)14-9-28)40(6,29-10-15-32(42)16-11-29)47(36)37(49)46-20-18-44(19-21-46)27-35(48)45-22-24-50-25-23-45/h8-17,26H,7,18-25,27H2,1-6H3/t39-,40+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | p53 and MDM2 proteins-interaction-inhibitor (chiral) (Compound 32) is an inhibitor of the interaction between p53 and MDM2 proteins. References: | |||||

p53 and MDM2 proteins-interaction-inhibitor chiral Dilution Calculator

p53 and MDM2 proteins-interaction-inhibitor chiral Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.361 mL | 6.805 mL | 13.6101 mL | 27.2201 mL | 34.0252 mL |
| 5 mM | 0.2722 mL | 1.361 mL | 2.722 mL | 5.444 mL | 6.805 mL |
| 10 mM | 0.1361 mL | 0.6805 mL | 1.361 mL | 2.722 mL | 3.4025 mL |
| 50 mM | 0.0272 mL | 0.1361 mL | 0.2722 mL | 0.5444 mL | 0.6805 mL |
| 100 mM | 0.0136 mL | 0.0681 mL | 0.1361 mL | 0.2722 mL | 0.3403 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
p53 and MDM2 proteins-interaction-inhibitor (chiral) is an inhibitor of the interaction between p53 and MDM2 proteins.
- BI 6015
Catalog No.:BCC6249
CAS No.:93987-29-2
- ACTB-1003
Catalog No.:BCC5587
CAS No.:939805-30-8
- PF-00562271
Catalog No.:BCC3684
CAS No.:939791-38-5
- [Ac-Tyr1,D-Phe2]GRF 1-29, amide (human)
Catalog No.:BCC5719
CAS No.:93965-89-0
- Fluvastatin Sodium
Catalog No.:BCC2317
CAS No.:93957-55-2
- Fluvastatin
Catalog No.:BCC1579
CAS No.:93957-54-1
- Toonaciliatin M
Catalog No.:BCN7881
CAS No.:93930-04-2
- Hirsutanonol 5-O-glucoside
Catalog No.:BCN4485
CAS No.:93915-36-7
- FIPI
Catalog No.:BCC7721
CAS No.:939055-18-2
- 9-Oxo-2,7-bisaboladien-15-oic acid
Catalog No.:BCN4484
CAS No.:93888-59-6
- Isochamaejasmine
Catalog No.:BCN3128
CAS No.:93859-63-3
- KU-0063794
Catalog No.:BCC2484
CAS No.:938440-64-3
- RG7112
Catalog No.:BCC1894
CAS No.:939981-39-2
- p53 and MDM2 proteins-interaction-inhibitor racemic
Catalog No.:BCC1831
CAS No.:939983-14-9
- Synephrine
Catalog No.:BCN6308
CAS No.:94-07-5
- Benzocaine
Catalog No.:BCC4636
CAS No.:94-09-7
- Propylparaben
Catalog No.:BCN8416
CAS No.:94-13-3
- Sodium 4-amiropparaty Hyalrate
Catalog No.:BCC3855
CAS No.:94-16-6
- Benzyl 4-hydroxybenzoate
Catalog No.:BCC8869
CAS No.:94-18-8
- Chlorpropamide
Catalog No.:BCC4647
CAS No.:94-20-2
- Tetracaine
Catalog No.:BCC9175
CAS No.:94-24-6
- Butylparaben
Catalog No.:BCN8418
CAS No.:94-26-8
- Benzyl nicotinate
Catalog No.:BCC8874
CAS No.:94-44-0
- 2-Amino-6-ethoxybenzothiazole
Catalog No.:BCC8541
CAS No.:94-45-1
Investigation of the inhibitory mechanism of apomorphine against MDM2-p53 interaction.[Pubmed:28400230]
Bioorg Med Chem Lett. 2017 Jun 1;27(11):2571-2574.
Mirror-image screening using d-proteins is a powerful approach to provide mirror-image structures of chiral natural products for drug screening. During the course of our screening study for novel MDM2-p53 interaction inhibitors, we identified that NPD6878 (R-(-)-apomorphine) inhibited both the native l-MDM2-l-p53 interaction and the mirror-image d-MDM2-d-p53 interaction at equipotent doses. In addition, both enantiomers of apomorphine showed potent inhibitory activity against the native MDM2-p53 interaction. In this study, we investigated the inhibitory mechanism of both enantiomers of apomorphine against the MDM2-p53 interaction. Achiral oxoapomorphine, which was converted from chiral apomorphines under aerobic conditions, served as the reactive species to form a covalent bond at Cys77 of MDM2, leading to the inhibitory effect against the binding to p53.
Organocatalytic, diastereo- and enantioselective synthesis of nonsymmetric cis-stilbene diamines: a platform for the preparation of single-enantiomer cis-imidazolines for protein-protein inhibition.[Pubmed:25017623]
J Org Chem. 2014 Aug 1;79(15):6913-38.
The finding by scientists at Hoffmann-La Roche that cis-imidazolines could disrupt the protein-protein interaction between p53 and MDM2, thereby inducing apoptosis in cancer cells, raised considerable interest in this scaffold over the past decade. Initial routes to these small molecules (i.e., Nutlin-3) provided only the racemic form, with enantiomers being enriched by chromatographic separation using high-pressure liquid chromatography (HPLC) and a chiral stationary phase. Reported here is the first application of an enantioselective aza-Henry approach to nonsymmetric cis-stilbene diamines and cis-imidazolines. Two novel mono(amidine) organocatalysts (MAM) were discovered to provide high levels of enantioselection (>95% ee) across a broad range of substrate combinations. Furthermore, the versatility of the aza-Henry strategy for preparing nonsymmetric cis-imidazolines is illustrated by a comparison of the roles of aryl nitromethane and aryl aldimine in the key step, which revealed unique substrate electronic effects providing direction for aza-Henry substrate-catalyst matching. This method was used to prepare highly substituted cis-4,5-diaryl imidazolines that project unique aromatic rings, and these were evaluated for MDM2-p53 inhibition in a fluorescence polarization assay. The diversification of access to cis-stilbene diamine-derived imidazolines provided by this platform should streamline their further development as chemical tools for disrupting protein-protein interactions.
Embryonic exposure to cis-bifenthrin enantioselectively induces the transcription of genes related to oxidative stress, apoptosis and immunotoxicity in zebrafish (Danio rerio).[Pubmed:23261506]
Fish Shellfish Immunol. 2013 Feb;34(2):717-23.
Cis-bifenthrin (cis-BF) is used widely for agricultural and non-agricultural purpose. Thus, cis-BF is one of the most frequently detected insecticides in the aquatic ecosystem. As a chiral pesticide, the commercial cis-BF contained two enantiomers including 1R-cis-BF and 1S-cis-BF. However, the difference in inducing oxidative stress, apoptosis and immunotoxicity by the two enantiomers in zebrafish still remains unclear. In the present study, the zebrafish were exposed to environmental concentrations of cis-BF, 1R-cis-BF and 1S-cis-BF during the embryos developmental stage. We observed that the mRNA levels of the most genes related to oxidative stress, apoptosis and immunotoxicity including Cu/Zn-superoxide dismutase (Cu/Zn-Sod), catalase (Cat), P53, murine double minute 2 (Mdm2), B-cell lymphoma/leukaemia-2 gene (Bcl2), Bcl2 associated X protein (Bax), apoptotic protease activating factor-1 (Apaf1), Caspase 9 (Cas9), Caspase 3 (Cas3), interleukin-1 beta (IL-1beta) and interleukin-8(Il-8) were much higher in 1S-cis-BF treated group than those in cis-BF or 1R-cis-BF treated ones, suggesting that 1S-cis-BF has higher risk to induced oxidative stress, apoptosis and immunotoxicity than 1R-cis-BF in zebrafish. The information presented in this study will help with elucidating the differences and environmental risk of the two enantiomers of cis-BF-induced toxicity in aquatic organisms.
A New Methodology for Incorporating Chiral Linkers into Stapled Peptides.[Pubmed:28388005]
Chembiochem. 2017 Jun 19;18(12):1066-1071.
Stapled peptides have arisen as a new class of chemical probe and potential therapeutic agents for modulating protein-protein interactions. Here, we report the first two-component i,i+7 stapling methodology that makes use of two orthogonal, on-resin stapling reactions to incorporate linkers bearing a chiral centre into a p53-derived stapled peptide. Post-stapling modifications to the chain were performed on-resin and enabled rapid access to various peptide derivatives from a single staple. The stapled peptides have increased helicity, protease stability and in vitro binding affinities to MDM2 compared to the equivalent unstapled peptide. This approach can be used to generate a library of diverse stapled peptides with different properties starting from a single stapled peptide, with scope for much greater functional diversity than that provided by existing stapling methodologies.
Meeting Organocatalysis with Drug Discovery: Asymmetric Synthesis of 3,3'-Spirooxindoles Fused with Tetrahydrothiopyrans as Novel p53-MDM2 Inhibitors.[Pubmed:26883465]
Org Lett. 2016 Mar 4;18(5):1028-31.
An organocatalytic enantioselective Michael-Michael cascade reaction is developed for the synthesis of chiral spirotetrahydrothiopyrans. This highly functionalized scaffold was assembled in moderate to good yield (55-74%) and excellent diastereo- and enantioselectivities (>30:1 dr, >/= 99% ee) with the creation of four consecutive stereogenic centers. The novel spiro-oxindole scaffold is validated as a new class of p53-MDM2 protein-protein interaction inhibitors with good antitumor activity.


