BenzocaineCAS# 94-09-7 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Capsaicin
Catalog No.:BCN1016
CAS No.:404-86-4
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 94-09-7 | SDF | Download SDF |
| PubChem ID | 2337 | Appearance | Powder |
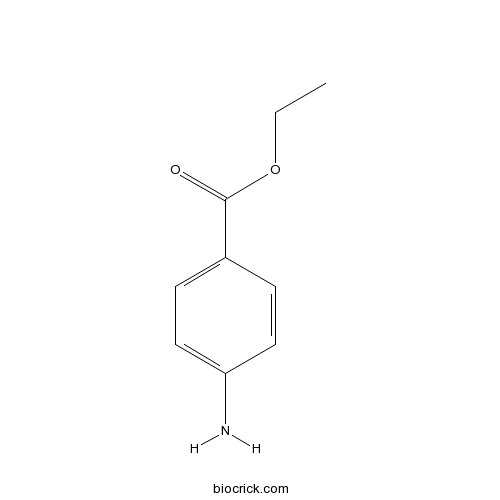
| Formula | C9H11NO2 | M.Wt | 165.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (605.36 mM) H2O : 2 mg/mL (12.11 mM; ultrasonic and warming and heat to 60°C) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | ethyl 4-aminobenzoate | ||
| SMILES | CCOC(=O)C1=CC=C(C=C1)N | ||
| Standard InChIKey | BLFLLBZGZJTVJG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H11NO2/c1-2-12-9(11)7-3-5-8(10)6-4-7/h3-6H,2,10H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Benzocaine Dilution Calculator

Benzocaine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0536 mL | 30.2682 mL | 60.5364 mL | 121.0727 mL | 151.3409 mL |
| 5 mM | 1.2107 mL | 6.0536 mL | 12.1073 mL | 24.2145 mL | 30.2682 mL |
| 10 mM | 0.6054 mL | 3.0268 mL | 6.0536 mL | 12.1073 mL | 15.1341 mL |
| 50 mM | 0.1211 mL | 0.6054 mL | 1.2107 mL | 2.4215 mL | 3.0268 mL |
| 100 mM | 0.0605 mL | 0.3027 mL | 0.6054 mL | 1.2107 mL | 1.5134 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Benzocaine is the ethyl ester of p-aminobenzoic acid (PABA), it is a local anesthetic commonly used as a topical pain reliever or in cough drops.
- Synephrine
Catalog No.:BCN6308
CAS No.:94-07-5
- p53 and MDM2 proteins-interaction-inhibitor racemic
Catalog No.:BCC1831
CAS No.:939983-14-9
- RG7112
Catalog No.:BCC1894
CAS No.:939981-39-2
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- BI 6015
Catalog No.:BCC6249
CAS No.:93987-29-2
- ACTB-1003
Catalog No.:BCC5587
CAS No.:939805-30-8
- PF-00562271
Catalog No.:BCC3684
CAS No.:939791-38-5
- [Ac-Tyr1,D-Phe2]GRF 1-29, amide (human)
Catalog No.:BCC5719
CAS No.:93965-89-0
- Fluvastatin Sodium
Catalog No.:BCC2317
CAS No.:93957-55-2
- Fluvastatin
Catalog No.:BCC1579
CAS No.:93957-54-1
- Toonaciliatin M
Catalog No.:BCN7881
CAS No.:93930-04-2
- Hirsutanonol 5-O-glucoside
Catalog No.:BCN4485
CAS No.:93915-36-7
- Propylparaben
Catalog No.:BCN8416
CAS No.:94-13-3
- Sodium 4-amiropparaty Hyalrate
Catalog No.:BCC3855
CAS No.:94-16-6
- Benzyl 4-hydroxybenzoate
Catalog No.:BCC8869
CAS No.:94-18-8
- Chlorpropamide
Catalog No.:BCC4647
CAS No.:94-20-2
- Tetracaine
Catalog No.:BCC9175
CAS No.:94-24-6
- Butylparaben
Catalog No.:BCN8418
CAS No.:94-26-8
- Benzyl nicotinate
Catalog No.:BCC8874
CAS No.:94-44-0
- 2-Amino-6-ethoxybenzothiazole
Catalog No.:BCC8541
CAS No.:94-45-1
- Piperine
Catalog No.:BCN1018
CAS No.:94-62-2
- N,N'-Bis(salicylidene)ethylenediamine
Catalog No.:BCC9064
CAS No.:94-93-9
- NVP-BHG712
Catalog No.:BCC3963
CAS No.:940310-85-0
- Suplatast Tosylate
Catalog No.:BCC4961
CAS No.:94055-76-2
The effect of benzocaine and ketoprofen gels on pain during fixed orthodontic appliance treatment: a randomised, double-blind, crossover trial.[Pubmed:27468593]
Aust Orthod J. 2016 May;32(1):64-72.
AIMS: To compare the analgesic effect of topical Benzocaine (5%) and ketoprofen (1.60 mg/mL) after 2 mm activation of 7 mm long delta loops used for maxillary en-masse orthodontic space closure. SUBJECTS AND METHODS: Twenty patients (seven males, 13 females, 15-25 years of age, mean age of 19.5 years) participated in a randomised crossover, double-blind trial. After appliance activation, participants were instructed to use analgesic gels and record pain perception at 2, 6, 24 hours and 2, 3 and 7 days (at 18.00 hrs), using a visual analogue scale ruler (VAS, 0-4). Each patient received all three gels (Benzocaine, ketoprofen, and a control (placebo)) randomly, but at three different appliance activation visits following a wash-over gap of one month. After the first day, the patients were instructed to repeat gel application twice a day at 10:00 and 18:00 hrs for three days. The recorded pain scores were subjected to non-parametric analysis. RESULTS: The highest pain was recorded at 2 and 6 hours. Pain scores were significantly different between the three groups (Kruskal-Wallis test, p < 0.01). The overall mean (SD) pain scores for the Benzocaine 5%, ketoprofen, and control (placebo) groups were 0.89 (0.41), 0.68 (0.34), and 1.15 (0.81), respectively. The pain scores were significantly different between the ketoprofen and control groups (mean difference = 0.47, p = 0.005). All groups demonstrated significant differences in pain scores at the six different time intervals (p < 0.05) and there was no gender difference (p > 0.05). CONCLUSION: A significant pain reduction was observed following the use of ketoprofen when tested against a control gel (placebo). The highest pain scores were experienced in patients administered the placebo and the lowest scores in patients who applied ketoprofen gel. Benzocaine had an effect mid-way between ketoprofen and the placebo. The highest pain scores were recorded 2 hours following force application, which decreased to the lowest scores after 7 days.
Topical Benzocaine and Methemoglobinemia.[Pubmed:27754990]
Am J Ther. 2017 Sep/Oct;24(5):e596-e598.
Methemoglobinemia can cause life-threatening hypoxia associated with cyanosis and dyspnea not responsive to oxygen. We present a case of recurrent methemoglobinemia because of occult use of topical Benzocaine to the vulva. A 47-year-old female with medical history of vulvar cancer and HIV undergoing chemoradiation was sent by the oncology clinic to the emergency department for worsening dyspnea, fatigue, hypoxia to 78% on room air, and gradual onset of cyanosis over the past week. A methemoglobin (MetHb) level was 49%. She received methylene blue, and repeat MetHb levels initially decreased but later increased to 56% despite continued treatment. Additional interviews with the patient revealed she was applying vagicaine (20% Benzocaine), an over the counter preparation to the vulvar area for analgesia, and she continued application while hospitalized. She received a total of 6 mg/kg methylene blue and underwent vaginal lavage with 60 mL of sterile saline and cleansed with soapy water. Cyanosis, hypoxia, and dyspnea resolved, and the MetHb level decreased to 5.4% on the day of discharge. Benzocaine is a frequent cause of iatrogenic methemoglobinemia. In this case, additional medication inquiries were helpful in making the diagnosis. Many patients do not consider over-the-counter medications to be potentially harmful. Methemoglobinemia from occult topical Benzocaine administration to the vulva is an uncommon exposure route. Occult medication use can be a source of methemoglobinemia.
Effect of 5% benzocaine gel on relieving pain caused by fixed orthodontic appliance activation. A double-blind randomized controlled trial.[Pubmed:27659276]
Orthod Craniofac Res. 2016 Nov;19(4):190-197.
AIM: To compare the effectiveness of 5% Benzocaine gel and placebo gel on reducing pain caused by fixed orthodontic appliance activation. SETTING AND SAMPLE POPULATION: Thirty subjects (15-25 years) undergoing fixed orthodontics. METHODS AND MATERIALS: A randomized, double-blind, placebo-controlled and cross-over clinical trial study was conducted. Subjects were asked to apply a placebo gel and 5% Benzocaine gel, exchangeable in two consecutive appointments, twice a day for 3 days and mark their level of pain on a VAS scale. The pain severity was evaluated by means of Mann-Whitney U-test for comparing two gel groups, Kruskal-Wallis nonparametric test for overall differences and post hoc test of Dunnett for paired multiple comparisons. p-value was assigned <0.05. RESULTS: The overall mean value of pain intensity for Benzocaine and placebo gels was 0.89 and 1.15, respectively. The Mann-Whitney U-test indicated that there was no significant difference between overall pain in both groups (mean difference = 0.258 p < 0.21). For both groups, pain intensity was significantly lower at 2, 6 and 24 h compared with pain experienced at days 2, 3 and 7. CONCLUSION: Benzocaine gel caused a decrease in pain perception at 2 h compared with placebo gel. Peak pain intensity was at 2 h for placebo gel and at 6 h for Benzocaine gel, followed by a decline in pain perception from that point to day 7 for both gels.


