Dinaciclib (SCH727965)Potent CDK inhibitor CAS# 779353-01-4 |
- CDK9 inhibitor 2
Catalog No.:BCC1466
CAS No.:1263369-28-3
- AZD-5438
Catalog No.:BCC3689
CAS No.:602306-29-6
- PHA-848125
Catalog No.:BCC3839
CAS No.:802539-81-7
- AT7519
Catalog No.:BCC2541
CAS No.:844442-38-2
- P276-00
Catalog No.:BCC4415
CAS No.:920113-03-7
- SB1317
Catalog No.:BCC1925
CAS No.:937270-47-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 779353-01-4 | SDF | Download SDF |
| PubChem ID | 46926350 | Appearance | Powder |
| Formula | C21H28N6O2 | M.Wt | 396.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SCH 727965 | ||
| Solubility | DMSO : ≥ 56 mg/mL (141.24 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
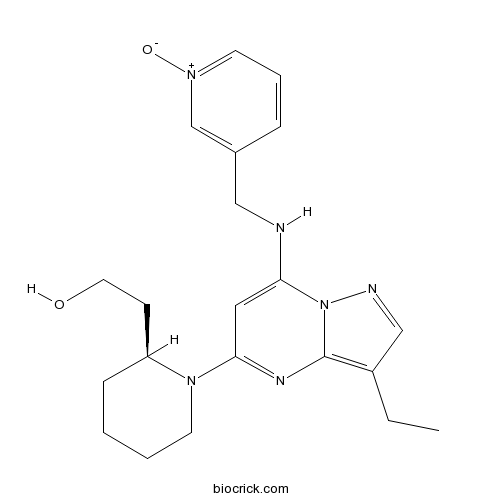
| Chemical Name | 2-[(2S)-1-[3-ethyl-7-[(1-oxidopyridin-1-ium-3-yl)methylamino]pyrazolo[1,5-a]pyrimidin-5-yl]piperidin-2-yl]ethanol | ||
| SMILES | CCC1=C2N=C(C=C(N2N=C1)NCC3=C[N+](=CC=C3)[O-])N4CCCCC4CCO | ||
| Standard InChIKey | PIMQWRZWLQKKBJ-SFHVURJKSA-N | ||
| Standard InChI | InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dinaciclib (SCH727965) is a novel and potent inhibitor of CDK for CDK2, CDK5, CDK1 and CDK9 with IC50 of 1 nM, 1 nM, 3 nM and 4 nM, respectively. | ||||||
| Targets | CDK2 | CDK5 | CDK1 | CDK9 | |||
| IC50 | 1 nM | 1 nM | 3 nM | 4 nM | |||
| Kinase experiment [1]: | |
| Cyclin/CDK kinase assay | Recombinant cyclin/CDK holoenzymes were purified from Sf9 cells engineered to produce baculoviruses that express a specific cyclin or CDK. Cyclin/CDK complexes were typically diluted to a final concentration of 50 μg/mL in a kinase reaction buffer containing 50 mmol/L Tris-HCl (pH 8.0), 10 mmol/L MgCl2, 1 mmol/L DTT, and 0.1 mmol/L sodium orthovanadate. For each kinase reaction, 1 μg of enzyme and 20 μL of a 2-μmol/L substrate solution (a biotinylated peptide derived from histone H1; Amersham) were mixed and combined with 10 μL of diluted SCH 727965. The reaction was started by the addition of 50 μL of 2 μmol/L ATP and 0.1 μCi of 33P-ATP. Kinase reactions were incubated for 1 hour at room temperature and were stopped by the addition of 0.1% Triton X-100, 1 mmol/L ATP, 5 mmol/L EDTA, and 5 mg/mL streptavidin-coated SPA beads. SPA beads were captured using a 96-well GF/B filter plate and a Filtermate universal harvester. Beads were washed twice with 2 mol/L NaCl and twice with 2 mol/L NaCl containing 1% phosphoric acid. The signal was then assayed using a Top-Count 96-well liquid scintillation counter. Dose-response curves were generated from duplicate, eight-point serial dilutions of inhibitory compounds. IC50 values were derived by nonlinear regression analysis. |
| Cell experiment [1]: | |
| Cell lines | A2780 cells |
| Preparation method | Limited solubility. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 2 h |
| Applications | SCH 727965 significantly abrogates phosphorylation of Rb on Ser 807/811 at concentrations >6.25 nmol/L and also leads to the generation of the p85 PARP cleavage product. 100 nmol/L SCH727965 treatment for 2 hours is effective in inducing suppression of Rb phosphorylation and caspase activation which can be detected up to 6 hours later. |
| Animal experiment [1]: | |
| Animal models | A2780 ovarian cancer mouse xenograft model |
| Dosage form | i.p. administration at 8, 16, 32, and 48 mg/kg daily |
| Application | SCH 727965 i.p. administration at 8, 16, 32, and 48 mg/kg daily for 10 days shows tumor inhibitionby 70%, 70%, 89%, and 96%, respectively. SCH 727965 is also well tolerated, and the maximum body weight loss in the highest dosage group is 5%. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. Parry D, Guzi T, Shanahan F et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010 Aug;9(8):2344-53. | |

Dinaciclib (SCH727965) Dilution Calculator

Dinaciclib (SCH727965) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5221 mL | 12.6107 mL | 25.2213 mL | 50.4426 mL | 63.0533 mL |
| 5 mM | 0.5044 mL | 2.5221 mL | 5.0443 mL | 10.0885 mL | 12.6107 mL |
| 10 mM | 0.2522 mL | 1.2611 mL | 2.5221 mL | 5.0443 mL | 6.3053 mL |
| 50 mM | 0.0504 mL | 0.2522 mL | 0.5044 mL | 1.0089 mL | 1.2611 mL |
| 100 mM | 0.0252 mL | 0.1261 mL | 0.2522 mL | 0.5044 mL | 0.6305 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dinaciclib is a potent cyclin-dependent kinase (CDK) inhibitor with IC50s for CDK2, CDK5, CDK1 and CDK9 at 1 nM, 1 nM, 3 nM, and 4 nM, respectively. [1] It is in phase I or II clinical trials for various cancers.
Dinaciclib interacts with the acetyl-lysine recognition site of bromodomains. [2] Inhibition of CDK 1 suppresses Rb phosphorylation, leading to cell cycle arrest and apoptosis. [3]
Dinaciclib is active against a broad spectrum of human tumor cell lines in vitro and in vivo. It has great potential to improve cancer chemotherapy.
References:
[1]Parry D., et al. (2010). Dinaciclib (SCH 727965), a Novel and Potent Cyclin-Dependent Kinase Inhibitor. Mol Cancer Ther (9): 2344- 2353.
[2]Martin, M. P., et al. (2013). Cyclin-dependent kinase inhibitor dinaciclib interacts with the acetyl-lysine recognition site of bromodomains. ACS Chemical Biology 8 (11): 2360–5.
[3]Payton M., et al. (2006). Discovery and Evaluation of Dual CDK1 and CDK2 Inhibitors. Cancer Res 66: 4299-4308.
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Beta-Lipotropin (1-10), porcine
Catalog No.:BCC1009
CAS No.:77875-68-4
- Isopulegol
Catalog No.:BCN4974
CAS No.:7786-67-6
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- (1R)-(+)-Alpha-Pinene
Catalog No.:BCC8275
CAS No.:7785-70-8
- Nelumol A
Catalog No.:BCN4749
CAS No.:77836-86-3
- GNF 5
Catalog No.:BCC3892
CAS No.:778277-15-9
- GNF 2
Catalog No.:BCC3891
CAS No.:778270-11-4
- Carasinol B
Catalog No.:BCN8226
CAS No.:777857-86-0
- Dehydrocrebanine
Catalog No.:BCN4328
CAS No.:77784-22-6
- Potassium phosphate monobasic
Catalog No.:BCC7583
CAS No.:7778-77-0
- 1,2-Dihydrotanshinquinone
Catalog No.:BCN2477
CAS No.:77769-21-2
- Longikaurin E
Catalog No.:BCN4329
CAS No.:77949-42-9
- Z-Glycinol
Catalog No.:BCC3095
CAS No.:77987-49-6
- Secologanin dimethyl acetal
Catalog No.:BCN4581
CAS No.:77988-07-9
- Mulberrofuran C
Catalog No.:BCN4032
CAS No.:77996-04-4
- Isophorone
Catalog No.:BCN8329
CAS No.:78-59-1
- Linalool
Catalog No.:BCN6339
CAS No.:78-70-6
- Zeylasterone
Catalog No.:BCN8057
CAS No.:78012-25-6
- 1,3-Diacetylvilasinin
Catalog No.:BCN4580
CAS No.:78012-28-9
- MMPX
Catalog No.:BCC6692
CAS No.:78033-08-6
- Fmoc-Lys(Fmoc)-OH
Catalog No.:BCC3521
CAS No.:78081-87-5
- (R)-(+)-8-Hydroxy-DPAT hydrobromide
Catalog No.:BCC6929
CAS No.:78095-19-9
- 2-Acetylfluorene
Catalog No.:BCC8516
CAS No.:781-73-7
Cyclin-dependent kinase inhibitor Dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models.[Pubmed:21768779]
Cancer Biol Ther. 2011 Oct 1;12(7):598-609. Epub 2011 Oct 1.
Pancreatic cancer is one of the most lethal of human malignancies, and potent therapeutic options are lacking. Inhibition of cell cycle progression through pharmacological blockade of cyclin-dependent kinases (CDK) has been suggested as a potential treatment option for human cancers with deregulated cell cycle control. Dinaciclib (SCH727965) is a novel small molecule multi-CDK inhibitor with low nanomolar potency against CDK1, CDK2, CDK5 and CDK9 that has shown favorable toxicity and efficacy in preliminary mouse experiments, and has been well tolerated in Phase I clinical trials. In the current study, the therapeutic efficacy of SCH727965 on human pancreatic cancer cells was tested using in vitro and in vivo model systems. Treatment with SCH727965 significantly reduced in vitro cell growth, motility and colony formation in soft agar of MIAPaCa-2 and Pa20C cells. These phenotypic changes were accompanied by marked reduction of phosphorylation of Retinoblastoma (Rb) and reduced activation of RalA. Single agent therapy with SCH727965 (40 mg/kg i.p. twice weekly) for 4 weeks significantly reduced subcutaneous tumor growth in 10/10 (100%) of tested low-passage human pancreatic cancer xenografts. Treatment of low passage pancreatic cancer xenografts with a combination of SCH727965 and gemcitabine was significantly more effective than either agent alone. Gene Set Enrichment Analysis identified overrepresentation of the Notch and Transforming Growth Factor-beta (TGF-beta) signaling pathways in the xenografts least responsive to SCH727965 treatment. Treatment with the cyclin-dependent kinase inhibitor SCH727965 alone or in combination is a highly promising novel experimental therapeutic strategy against pancreatic cancer.
Dinaciclib (SCH727965) inhibits the unfolded protein response through a CDK1- and 5-dependent mechanism.[Pubmed:24362465]
Mol Cancer Ther. 2014 Mar;13(3):662-74.
Evidence implicating dysregulation of the IRE1/XBP-1s arm of the unfolded protein response (UPR) in cancer pathogenesis (e.g., multiple myeloma) has prompted the development of IRE1 RNase inhibitors. Here, effects of cyclin-dependent kinase (CDK) inhibitor SCH727965 (dinaciclib) on the IRE1 arm of the UPR were examined in human leukemia and myeloma cells. Exposure of cells to extremely low (e.g., nmol/L) concentrations of SCH727965, a potent inhibitor of CDKs 1/2/5/9, diminished XBP-1s and Grp78 induction by the endoplasmic reticulum (ER) stress-inducers thapsigargin and tunicamycin, while sharply inducing cell death. SCH727965, in contrast to IRE1 RNase inhibitors, inhibited the UPR in association with attenuation of XBP-1s nuclear localization and accumulation rather than transcription, translation, or XBP-1 splicing. Notably, in human leukemia cells, CDK1 and 5 short hairpin RNA (shRNA) knockdown diminished Grp78 and XBP-1s upregulation while increasing thapsigargin lethality, arguing for a functional role for CDK1/5 in activation of the cytoprotective IRE1/XBP-1s arm of the UPR. In contrast, CDK9 or 2 inhibitors or shRNA knockdown failed to downregulate XBP-1s or Grp78. Furthermore, IRE1, XBP-1, or Grp78 knockdown significantly increased thapsigargin lethality, as observed with CDK1/5 inhibition/knockdown. Finally, SCH727965 diminished myeloma cell growth in vivo in association with XBP-1s downregulation. Together, these findings demonstrate that SCH727965 acts at extremely low concentrations to attenuate XBP-1s nuclear accumulation and Grp78 upregulation in response to ER stress inducers. They also highlight a link between specific components of the cell-cycle regulatory apparatus (e.g., CDK1/5) and the cytoprotective IRE1/XBP-1s/Grp78 arm of the UPR that may be exploited therapeutically in UPR-driven malignancies.


