LinaloolCAS# 78-70-6 |
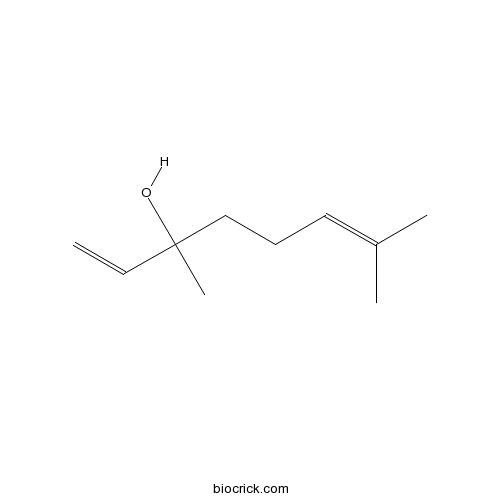
- (-)-Linalool
Catalog No.:BCN9893
CAS No.:126-91-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78-70-6 | SDF | Download SDF |
| PubChem ID | 6549 | Appearance | Colorless liquid |
| Formula | C10H18O | M.Wt | 154.25 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Linalol; Linalyl alcohol; Linanool | ||
| Solubility | Soluble in ethanol; insoluble in water | ||
| Chemical Name | 3,7-dimethylocta-1,6-dien-3-ol | ||
| SMILES | CC(=CCCC(C)(C=C)O)C | ||
| Standard InChIKey | CDOSHBSSFJOMGT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H18O/c1-5-10(4,11)8-6-7-9(2)3/h5,7,11H,1,6,8H2,2-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Linalool, is a competitive antagonist of NMDA receptors, which has anti-inflammatory, antinociceptive, anti-anxiety, local anaesthetic, anti-leishmanicidal, and insecticidal properties. Linalool has dose-dependent marked sedative effects at the central nervous system (CNS), including hypnotic, anticonvulsant and hypothermic properties, it also has an inhibitory effect on the acetylcholine (ACh) release and on the channel open time in the mouse neuromuscular junction. The purified linalool fraction is only inhibitory for C. albicans. |
| Targets | IL Receptor | TNF-α | IkB | p38MAPK | JNK | IKK |
| In vitro | Antileishmanial activity of a linalool-rich essential oil from Croton cajucara.[Pubmed: 12760864 ]Antimicrob. Agents Ch., 2003, 47(6):1895-901.The in vitro leishmanicidal effects of a Linalool-rich essential oil from the leaves of Croton cajucara against Leishmania amazonensis were investigated.
Antimicrobial activity of Croton cajucara Benth linalool-rich essential oil on artificial biofilms and planktonic microorganisms.[Pubmed: 15720570 ]Oral Microbiol Immunol. 2005 Apr;20(2):101-5.We have previously demonstrated that a Linalool-rich essential oil from Croton cajucara Benth presents leishmanicidal activity.
Linalool modifies the nicotinic receptor-ion channel kinetics at the mouse neuromuscular junction.[Pubmed: 10887049 ]Pharmacol Res. 2000 Aug;42(2):177-82.Linalool is a monoterpene compound reported to be a major component of essential oils in various aromatic species. Several Linalool-producing species are used in traditional medical systems. Among these is Aeolanthus suaveolens G. Dom (Labiatae) which is used as an anticonvulsant in the Brazilian Amazon. Psychopharmacological in vivo evaluation of Linalool showed that this compound has dose-dependent marked sedative effects at the central nervous system (CNS), including hypnotic, anticonvulsant and hypothermic properties. It has been suggested that these neurochemical effects might be ascribed to the local anaesthetic activity of Linalool.
Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells.[Pubmed: 19049815 ]Food Chem Toxicol. 2009 Jan;47(1):260-6.We studied the protective effect of monoterpenes myrcene, eucalyptol and Linalool against t-butyl hydroperoxide (t-BOOH) induced genotoxicity in reverse mutation assay with Escherichia coli WP2 IC185 strain and its oxyR mutant IC202, and with the comet assay in human hepatoma HepG2 and human B lymphoid NC-NC cells.
|
| In vivo | Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model.[Pubmed: 23228323 ]J Surg Res. 2013 Mar;180(1):e47-54.Inflammation, characterized by redness, swelling, pain and a sensation of heat, is one of the body's self-defense systems. Although the inflammation response has an important role in host survival, it also leads to chronic inflammatory diseases. Linalool is a natural compound of the essential oils in several aromatic plants species. It possesses anti-inflammatory, antinociceptive, and other bioactive properties. In the present study, we investigated the protective effects of Linalool on inflammation in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells and an LPS-induced in vivo lung injury model. Linalool attenuated the production of LPS-induced tumor necrosis-α and interleukin-6 both in vitro and in vivo. Furthermore, phosphorylation of IκBα protein, p38, c-Jun terminal kinase, and extracellular signal-regulated kinase in LPS-stimulated RAW 264.7 cells was blocked by Linalool. Our in vivo study also found that Linalool attenuated lung histopathologic changes in mouse models. CONCLUSIONS: The results suggest that Linalool inhibits inflammation both in vitro and in vivo, and may be a potential therapeutic candidate for the treatment of inflammatory diseases. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice.[Pubmed: 19962290 ]Phytomedicine. 2010 Jul;17(8-9):679-83.Aromatherapy uses essential oils (EOs) for several medical purposes, including relaxation. The association between the use of aromas and a decrease in anxiety could be a valuable instrument in managing anxiety in an ever increasing anxiogenic daily life style. Linalool is a monoterpene commonly found as the major volatile component of EOs in several aromatic plant species.
|
| Cell Research | Linalool Induces Cell Cycle Arrest and Apoptosis in Leukemia Cells and Cervical Cancer Cells through CDKIs[Pubmed: 26703569]Int J Mol Sci. 2015 Dec; 16(12): 28169–28179.
Cell lines:U937 cells |
| Animal Research | Effect of linalool on morphine tolerance and dependence in mice.[Pubmed: 22318903 ]Phytother Res. 2012 Sep;26(9):1399-404.Animal Models:Adult male albino mice |

Linalool Dilution Calculator

Linalool Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.483 mL | 32.4149 mL | 64.8298 mL | 129.6596 mL | 162.0746 mL |
| 5 mM | 1.2966 mL | 6.483 mL | 12.966 mL | 25.9319 mL | 32.4149 mL |
| 10 mM | 0.6483 mL | 3.2415 mL | 6.483 mL | 12.966 mL | 16.2075 mL |
| 50 mM | 0.1297 mL | 0.6483 mL | 1.2966 mL | 2.5932 mL | 3.2415 mL |
| 100 mM | 0.0648 mL | 0.3241 mL | 0.6483 mL | 1.2966 mL | 1.6207 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isophorone
Catalog No.:BCN8329
CAS No.:78-59-1
- Mulberrofuran C
Catalog No.:BCN4032
CAS No.:77996-04-4
- Secologanin dimethyl acetal
Catalog No.:BCN4581
CAS No.:77988-07-9
- Z-Glycinol
Catalog No.:BCC3095
CAS No.:77987-49-6
- Longikaurin E
Catalog No.:BCN4329
CAS No.:77949-42-9
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Beta-Lipotropin (1-10), porcine
Catalog No.:BCC1009
CAS No.:77875-68-4
- Isopulegol
Catalog No.:BCN4974
CAS No.:7786-67-6
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- (1R)-(+)-Alpha-Pinene
Catalog No.:BCC8275
CAS No.:7785-70-8
- Nelumol A
Catalog No.:BCN4749
CAS No.:77836-86-3
- Zeylasterone
Catalog No.:BCN8057
CAS No.:78012-25-6
- 1,3-Diacetylvilasinin
Catalog No.:BCN4580
CAS No.:78012-28-9
- MMPX
Catalog No.:BCC6692
CAS No.:78033-08-6
- Fmoc-Lys(Fmoc)-OH
Catalog No.:BCC3521
CAS No.:78081-87-5
- (R)-(+)-8-Hydroxy-DPAT hydrobromide
Catalog No.:BCC6929
CAS No.:78095-19-9
- 2-Acetylfluorene
Catalog No.:BCC8516
CAS No.:781-73-7
- Aztreonam
Catalog No.:BCC2557
CAS No.:78110-38-0
- Okadaic acid
Catalog No.:BCC2464
CAS No.:78111-17-8
- DAMGO
Catalog No.:BCC6958
CAS No.:78123-71-4
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- CDPPB
Catalog No.:BCC7610
CAS No.:781652-57-1
- 4-Hydroxyisoleucine
Catalog No.:BCN1211
CAS No.:781658-23-9
Linalool modifies the nicotinic receptor-ion channel kinetics at the mouse neuromuscular junction.[Pubmed:10887049]
Pharmacol Res. 2000 Aug;42(2):177-82.
Linalool is a monoterpene compound reported to be a major component of essential oils in various aromatic species. Several Linalool-producing species are used in traditional medical systems. Among these is Aeolanthus suaveolens G. Dom (Labiatae) which is used as an anticonvulsant in the Brazilian Amazon. Psychopharmacological in vivo evaluation of Linalool showed that this compound has dose-dependent marked sedative effects at the central nervous system (CNS), including hypnotic, anticonvulsant and hypothermic properties. It has been suggested that these neurochemical effects might be ascribed to the local anaesthetic activity of Linalool. The present study reports an inhibitory effect of Linalool on the acetylcholine (ACh) release and on the channel open time in the mouse neuromuscular junction. These findings could provide a rational basis to confirm the traditional medical use of Linalool-producing plant species. Indeed, our data demonstrate some interactions in the modulation of the ACh release at the mouse neuromuscular junction, which are well correlated with the suggested molecular mechanisms. Linalool induced a reduction of the ACh-evoked release. The possibility that this effect could be ascribed to some interaction with pre-synaptic function is noteworthy. Moreover, the inhibitory effect induced on the kinetics of the miniature end-plate current decay demonstrates a local anaesthetic action, either on the voltage or on the receptor-activated channels.
Antimicrobial activity of Croton cajucara Benth linalool-rich essential oil on artificial biofilms and planktonic microorganisms.[Pubmed:15720570]
Oral Microbiol Immunol. 2005 Apr;20(2):101-5.
We have previously demonstrated that a Linalool-rich essential oil from Croton cajucara Benth presents leishmanicidal activity. In the present study, we demonstrate that this essential oil inhibits the growth of reference samples of Candida albicans, Lactobacillus casei, Staphylococcus aureus, Streptococcus sobrinus, Porphyromonas gingivalis and Streptococcus mutans cell suspensions, all of them associated with oral cavity disease. The purified Linalool fraction was only inhibitory for C. albicans. Microbes of saliva specimens from human individuals with fixed orthodontic appliances, as well as the reference strains, were used to construct an artificial biofilm which was exposed to Linalool or to the essential oil. As in microbial suspensions, the essential oil was toxic for all the microorganisms, while the purified Linalool fraction mainly inhibited the growth of C. albicans. The compounds of the essential oil were separated by thin layer chromatography and exposed to the above-cited microorganisms. In this analysis, the proliferation of the bacterial cells was inhibited by still uncharacterized molecules, and Linalool was confirmed as the antifungal component of the essential oil. The effects of Linalool on the cell biology of C. albicans were evaluated by electron microscopy, which showed that Linalool induced a reduction in cell size and abnormal germination. Neither the crude essential oil nor the purified Linalool fraction is toxic to mammalian cells, which suggests that the essential oil or its purified components may be useful to control the microbial population in patients with fixed orthodontic appliances.
Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells.[Pubmed:19049815]
Food Chem Toxicol. 2009 Jan;47(1):260-6.
We studied the protective effect of monoterpenes myrcene, eucalyptol and Linalool against t-butyl hydroperoxide (t-BOOH) induced genotoxicity in reverse mutation assay with Escherichia coli WP2 IC185 strain and its oxyR mutant IC202, and with the comet assay in human hepatoma HepG2 and human B lymphoid NC-NC cells. The monoterpenes were tested in concentration ranges 0.05-1.5 mg/plate and 0.01-1.0 microg/ml in bacteria and mammalian cells, respectively. Suppression of t-BOOH induced mutagenesis was detected only in IC202 strain, and correlated with the observed inhibition of lipid peroxidation by the three monoterpenes. Linalool and myrcene strongly suppressed t-BOOH induced mutagenesis. Eucalyptol, in addition to moderate suppression of t-BOOH induced mutagenesis, suppressed also spontaneous mutagenesis. In NC-NC cells Linalool and myrcene reduced t-BOOH induced DNA damage by about 50% at 0.01 microg/ml, while eucalyptol was less efficient (about 50% reduction at 1.0 microg/ml). In HepG2 cells Linalool and eucalyptol reduced DNA damage by 30% and 40%, respectively, while myrcene was ineffective. The repair of t-BOOH induced DNA damage, studied in HepG2 cells, was not affected by monoterpenes. The results indicate that Linalool, eucalyptol and myrcene have substantial protective effect against oxidant induced genotoxicity, which is predominately mediated by their radical scavenging activity.
Antileishmanial activity of a linalool-rich essential oil from Croton cajucara.[Pubmed:12760864]
Antimicrob Agents Chemother. 2003 Jun;47(6):1895-901.
The in vitro leishmanicidal effects of a Linalool-rich essential oil from the leaves of Croton cajucara against Leishmania amazonensis were investigated. Morphological changes in L. amazonensis promastigotes treated with 15 ng of essential oil per ml were observed by transmission electron microscopy; leishmanial nuclear and kinetoplast chromatin destruction, followed by cell lysis, was observed within 1 h. Pretreatment of mouse peritoneal macrophages with 15 ng of essential oil per ml reduced by 50% the interaction between these macrophages and L. amazonensis, with a concomitant increase by 220% in the level of nitric oxide production by the infected macrophages. Treatment of preinfected macrophages with 15 ng of essential oil per ml reduced by 50% the interaction between these cells and the parasites, which led to a 60% increase in the amount of nitric oxide produced by the preinfected macrophages. These results provide new perspectives on the development of drugs with activities against Leishmania, as Linalool-rich essential oil is a strikingly potent leishmanicidal plant extract (50% lethal doses, 8.3 ng/ml for promastigotes and 8.7 ng/ml for amastigotes) which inhibited the growth of L. amazonensis promastigotes at very low concentrations (MIC, 85.0 pg/ml) and which presented no cytotoxic effects against mammalian cells.
Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice.[Pubmed:19962290]
Phytomedicine. 2010 Jul;17(8-9):679-83.
Aromatherapy uses essential oils (EOs) for several medical purposes, including relaxation. The association between the use of aromas and a decrease in anxiety could be a valuable instrument in managing anxiety in an ever increasing anxiogenic daily life style. Linalool is a monoterpene commonly found as the major volatile component of EOs in several aromatic plant species. Adding to previously reported sedative effects of inhaled Linalool, the aim of this study was to investigate the effects of inhaled Linalool on anxiety, aggressiveness and social interaction in mice. Additionally, we investigated the effects of inhaled Linalool on the acquisition phase of a step-down memory task in mice. Inhaled Linalool showed anxiolytic properties in the light/dark test, increased social interaction and decreased aggressive behavior; impaired memory was only seen the higher dose of Linalool. These results strengthen the suggestion that inhaling Linalool rich essential oils can be useful as a mean to attain relaxation and counteract anxiety.
Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model.[Pubmed:23228323]
J Surg Res. 2013 Mar;180(1):e47-54.
BACKGROUND: Inflammation, characterized by redness, swelling, pain and a sensation of heat, is one of the body's self-defense systems. Although the inflammation response has an important role in host survival, it also leads to chronic inflammatory diseases. Linalool is a natural compound of the essential oils in several aromatic plants species. It possesses anti-inflammatory, antinociceptive, and other bioactive properties. In the present study, we investigated the protective effects of Linalool on inflammation in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells and an LPS-induced in vivo lung injury model. METHODS: We evaluated the effects of Linalool on LPS-induced production of inflammatory mediators in Raw 264.7 murine macrophages by enzyme-linked immunosorbent assay and Western blot. To confirm the anti-inflammatory activity of Linalool in vivo, we induced an acute lung injury in an LPS-induced mouse model. RESULTS: Linalool attenuated the production of LPS-induced tumor necrosis-alpha and interleukin-6 both in vitro and in vivo. Furthermore, phosphorylation of IkappaBalpha protein, p38, c-Jun terminal kinase, and extracellular signal-regulated kinase in LPS-stimulated RAW 264.7 cells was blocked by Linalool. Our in vivo study also found that Linalool attenuated lung histopathologic changes in mouse models. CONCLUSIONS: The results suggest that Linalool inhibits inflammation both in vitro and in vivo, and may be a potential therapeutic candidate for the treatment of inflammatory diseases.


