AztreonamCAS# 78110-38-0 |
- Nateglinide
Catalog No.:BCC5005
CAS No.:105816-04-4
- ML133 HCl
Catalog No.:BCC5006
CAS No.:1222781-70-5
- Dronedarone
Catalog No.:BCN2176
CAS No.:141626-36-0
- Gliclazide
Catalog No.:BCC5002
CAS No.:21187-98-4
- Tolbutamide
Catalog No.:BCC5001
CAS No.:64-77-7
- Nicorandil
Catalog No.:BCC5004
CAS No.:65141-46-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78110-38-0 | SDF | Download SDF |
| PubChem ID | 5742832 | Appearance | Powder |
| Formula | C13H17N5O8S2 | M.Wt | 435.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Azactam | ||
| Solubility | DMSO : 50 mg/mL (114.83 mM; Need ultrasonic) H2O : 10 mg/mL (22.97 mM; Need ultrasonic) | ||
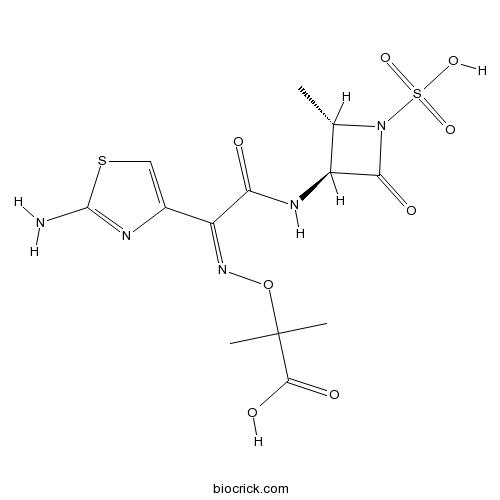
| Chemical Name | 2-[(Z)-[1-(2-amino-1,3-thiazol-4-yl)-2-[[(2S,3S)-2-methyl-4-oxo-1-sulfoazetidin-3-yl]amino]-2-oxoethylidene]amino]oxy-2-methylpropanoic acid | ||
| SMILES | C[C@H]1[C@H](NC(=O)C(=N/OC(C)(C)C(O)=O)c2csc([NH3+])n2)C(=O)N1[S]([O-])(=O)=O | ||
| Standard InChIKey | WZPBZJONDBGPKJ-VEHQQRBSSA-N | ||
| Standard InChI | InChI=1S/C13H17N5O8S2/c1-5-7(10(20)18(5)28(23,24)25)16-9(19)8(6-4-27-12(14)15-6)17-26-13(2,3)11(21)22/h4-5,7H,1-3H3,(H2,14,15)(H,16,19)(H,21,22)(H,23,24,25)/b17-8-/t5-,7-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Aztreonam is a synthetic monocyclic beta-lactam antibiotic, which has a very high affinity for penicillin-binding protein 3 (PBP-3).
Target: Penicillin-binding proteins 3 (PBP-3)
Aztreonam is a synthetic monocyclic beta-lactam antibiotic (a monobactam), with the nucleus based on a simpler monobactam isolated from Chromobacterium violaceum. It was approved by the U.S. Food and Drug Administration in 1986. It is resistant to some beta-lactamases, but is inactivated by extended-spectrum beta-lactamases. Aztreonam has no useful activity against gram-positive or anaerobic microorganisms
Aztreonam is similar in action to penicillin. It inhibits mucopeptide synthesis in the bacterial cell wall, thereby blocking peptidoglycan crosslinking. It has a very high affinity for penicillin-binding protein 3 (PBP-3) and mild affinity for PBP-1a. Aztreonam binds the penicillin-binding proteins of gram-positive and anaerobic bacteria very poorly and is largely ineffective against them. Aztreonam is bactericidal but less so than some of the cephalosporins References: | |||||

Aztreonam Dilution Calculator

Aztreonam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2966 mL | 11.4829 mL | 22.9658 mL | 45.9316 mL | 57.4145 mL |
| 5 mM | 0.4593 mL | 2.2966 mL | 4.5932 mL | 9.1863 mL | 11.4829 mL |
| 10 mM | 0.2297 mL | 1.1483 mL | 2.2966 mL | 4.5932 mL | 5.7415 mL |
| 50 mM | 0.0459 mL | 0.2297 mL | 0.4593 mL | 0.9186 mL | 1.1483 mL |
| 100 mM | 0.023 mL | 0.1148 mL | 0.2297 mL | 0.4593 mL | 0.5741 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Aztreonam is the first totally synthetic monocyclic β-lactam antibiotic.
- 2-Acetylfluorene
Catalog No.:BCC8516
CAS No.:781-73-7
- (R)-(+)-8-Hydroxy-DPAT hydrobromide
Catalog No.:BCC6929
CAS No.:78095-19-9
- Fmoc-Lys(Fmoc)-OH
Catalog No.:BCC3521
CAS No.:78081-87-5
- MMPX
Catalog No.:BCC6692
CAS No.:78033-08-6
- 1,3-Diacetylvilasinin
Catalog No.:BCN4580
CAS No.:78012-28-9
- Zeylasterone
Catalog No.:BCN8057
CAS No.:78012-25-6
- Linalool
Catalog No.:BCN6339
CAS No.:78-70-6
- Isophorone
Catalog No.:BCN8329
CAS No.:78-59-1
- Mulberrofuran C
Catalog No.:BCN4032
CAS No.:77996-04-4
- Secologanin dimethyl acetal
Catalog No.:BCN4581
CAS No.:77988-07-9
- Z-Glycinol
Catalog No.:BCC3095
CAS No.:77987-49-6
- Longikaurin E
Catalog No.:BCN4329
CAS No.:77949-42-9
- Okadaic acid
Catalog No.:BCC2464
CAS No.:78111-17-8
- DAMGO
Catalog No.:BCC6958
CAS No.:78123-71-4
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- CDPPB
Catalog No.:BCC7610
CAS No.:781652-57-1
- 4-Hydroxyisoleucine
Catalog No.:BCN1211
CAS No.:781658-23-9
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
- Nirtetralin
Catalog No.:BCN3755
CAS No.:78185-63-4
- 20(S)-Ginsenoside Rh2
Catalog No.:BCN1070
CAS No.:78214-33-2
- Paroxetine HCl
Catalog No.:BCC5054
CAS No.:78246-49-8
- [Orn5]-URP
Catalog No.:BCC5985
CAS No.:782485-03-4
- Hydroxysafflor yellow A
Catalog No.:BCN1049
CAS No.:78281-02-4
- Nepafenac
Catalog No.:BCC1258
CAS No.:78281-72-8
Prediction of in vivo and in vitro infection model results using a semimechanistic model of avibactam and aztreonam combination against multidrug resistant organisms.[Pubmed:28145085]
CPT Pharmacometrics Syst Pharmacol. 2017 Mar;6(3):197-207.
The combination of Aztreonam-avibactam is active against multidrug-resistant Enterobacteriaceae that express metallo-beta-lactamases. A complex synergistic interaction exists between Aztreonam and avibactam bactericidal activities that have not been quantitatively explored. A two-state semimechanistic pharmacokinetic/pharmacodynamic (PK/PD) logistic growth model was developed to account for antimicrobial activities in the combination of bacteria-mediated degradation of Aztreonam and the inhibition of Aztreonam degradation by avibactam. The model predicted that changing regimens of 2 g Aztreonam plus 0.375 and 0.6 g avibactam as a 1-hour infusion were qualitatively similar to that observed from in vivo murine thigh infection and hollow-fiber infection models previously reported in the literature with 24-hour log kill >/=1. The current approach to characterize the effect of avibactam in enhancing Aztreonam activity from time-kill study was accomplished by shifting the half-maximal effective concentration (EC50 ) of Aztreonam in increasing avibactam concentration using a nonlinear equation as a function of avibactam concentration, providing a framework for translational predictions.
Susceptibility to cephalosporin combinations and aztreonam/avibactam among third-generation cephalosporin-resistant Enterobacteriaceae recovered on hospital admission.[Pubmed:27939093]
Int J Antimicrob Agents. 2017 Feb;49(2):239-242.
As part of the multicentre Antibiotic Therapy Optimisation Study (ATHOS), minimum inhibitory concentrations (MICs) were determined for cephalosporins alone and in combination with the beta-lactamase inhibitors tazobactam, clavulanic acid and avibactam against third-generation cephalosporin-resistant Escherichia coli, Klebsiella spp. and Enterobacter spp. isolates collected in German hospitals. MIC50/90 values were 0.25-4 mg/L for cefepime/tazobactam, 0.25-2 mg/L for ceftazidime/avibactam, 0.125-0.5 mg/L for ceftaroline/avibactam, 0.5-4 mg/L for cefpodoxime/clavulanic acid and 0.25-1 mg/L for Aztreonam/avibactam, depending on the underlying resistance mechanism and organism. Based on in vitro testing, beta-lactam antibiotics play an important role in the treatment of infections due to beta-lactamase-producing organisms.
Can Ceftazidime-Avibactam and Aztreonam Overcome beta-Lactam Resistance Conferred by Metallo-beta-Lactamases in Enterobacteriaceae?[Pubmed:28167541]
Antimicrob Agents Chemother. 2017 Mar 24;61(4). pii: AAC.02243-16.
Based upon knowledge of the hydrolytic profile of major beta-lactamases found in Gram-negative bacteria, we tested the efficacy of the combination of ceftazidime-avibactam (CAZ-AVI) with Aztreonam (ATM) against carbapenem-resistant enteric bacteria possessing metallo-beta-lactamases (MBLs). Disk diffusion and agar-based antimicrobial susceptibility testing were initially performed to determine the in vitro efficacy of a unique combination of CAZ-AVI and ATM against 21 representative Enterobacteriaceae isolates with a complex molecular background that included blaIMP, blaNDM, blaOXA-48, blaCTX-M, blaAmpC, and combinations thereof. Time-kill assays were conducted, and the in vivo efficacy of this combination was assessed in a murine neutropenic thigh infection model. By disk diffusion assay, all 21 isolates were resistant to CAZ-AVI alone, and 19/21 were resistant to ATM. The in vitro activity of CAZ-AVI in combination with ATM against diverse Enterobacteriaceae possessing MBLs was demonstrated in 17/21 isolates, where the zone of inhibition was >/=21 mm. All isolates demonstrated a reduction in CAZ-AVI agar dilution MICs with the addition of ATM. At 2 h, time-kill assays demonstrated a >/=4-log10-CFU decrease for all groups that had CAZ-AVI with ATM (8 mug/ml) added, compared to the group treated with CAZ-AVI alone. In the murine neutropenic thigh infection model, an almost 4-log10-CFU reduction was noted at 24 h for CAZ-AVI (32 mg/kg every 8 h [q8h]) plus ATM (32 mg/kg q8h) versus CAZ-AVI (32 mg/kg q8h) alone. The data presented herein require us to carefully consider this new therapeutic combination to treat infections caused by MBL-producing Enterobacteriaceae.
Repurposing an old drug: aztreonam as a new treatment strategy for gonorrhoea.[Pubmed:28137938]
J Antimicrob Chemother. 2017 May 1;72(5):1466-1468.
Objectives: To determine whether Aztreonam is still an effective drug for the treatment of gonorrhoea. Methods: Observational study of patients with gonorrhoea diagnosed by urine multiplex PCR, with a past medical history of allergy to beta-lactams or relapse after treatment with a third-generation cephalosporin. Patients received a single 1 g dose of Aztreonam in accordance with the manufacturer's instructions. Results: Five patients (four males, one female) were enrolled, comprising two who were allergic to beta-lactams and three previously treated with cephalosporins who relapsed. Median age was 38 years (range 23-51). Following treatment with Aztreonam all were cured without any adverse event. All the men were free of symptoms, and the woman tested negative for gonorrhoea 1 month after treatment. Conclusion: Aztreonam appears to be an effective alternative to cephalosporins in the treatment of uncomplicated gonorrhoea, particularly when patients are suspected of being infected by strains with reduced susceptibility to ceftriaxone or are known to be allergic to penicillin.


