DronedaroneCAS# 141626-36-0 |
- Dofetilide
Catalog No.:BCC3770
CAS No.:115256-11-6
- Repaglinide
Catalog No.:BCC2504
CAS No.:135062-02-1
- NS309
Catalog No.:BCC1809
CAS No.:18711-16-5
- TRAM-34
Catalog No.:BCC1122
CAS No.:289905-88-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 141626-36-0 | SDF | Download SDF |
| PubChem ID | 208898 | Appearance | Powder |
| Formula | C31H44N2O5S | M.Wt | 556.76 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (179.61 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
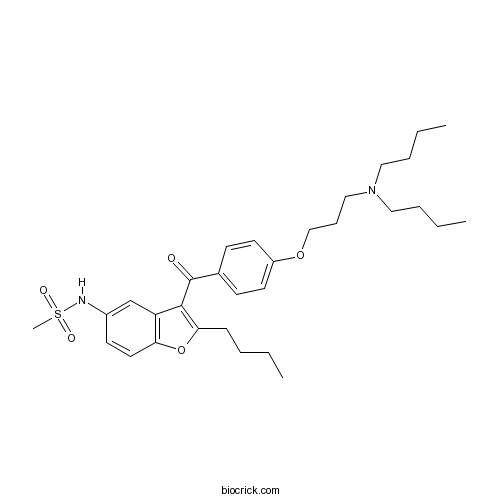
| Chemical Name | N-[2-butyl-3-[4-[3-(dibutylamino)propoxy]benzoyl]-1-benzofuran-5-yl]methanesulfonamide | ||
| SMILES | CCCCC1=C(C2=C(O1)C=CC(=C2)NS(=O)(=O)C)C(=O)C3=CC=C(C=C3)OCCCN(CCCC)CCCC | ||
| Standard InChIKey | ZQTNQVWKHCQYLQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C31H44N2O5S/c1-5-8-12-29-30(27-23-25(32-39(4,35)36)15-18-28(27)38-29)31(34)24-13-16-26(17-14-24)37-22-11-21-33(19-9-6-2)20-10-7-3/h13-18,23,32H,5-12,19-22H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dronedarone is a derivative of amiodarone which is classified as a Class III antiarrhythmic agent. It shows rate-dependent inhibition of the rapid Na+ current, inhibits α and β-adrenergic receptors like Class II agents, exhibits blockade of K+ outward currents as the main mechanism of action of Class III, and effectively block slow Ca2+ inward currents (Class IV). |
| Targets | Calcium Channel | Sodium Channel | Potassium Channel |
| In vivo | Dronedarone and renal impairment: evaluation of Spanish postmarketing reports and review of literature.[Pubmed: 25967281]Expert Opin Drug Saf. 2015 Jun;14(6):807-13.Renal impairment associated with Dronedarone use is hardly known. Our aim is to describe the characteristics of spontaneous reports involving renal adverse reactions with use of Dronedarone.
The role of dronedarone in the treatment of atrial fibrillation/flutter in the aftermath of PALLAS.[Pubmed: 24821656]Curr Cardiol Rev. 2014 Nov;10(4):303-8.Dronedarone is an amiodarone analog that differs structurally from amiodarone in that the iodine moiety was removed and a methane-sulfonyl group was added. These modifications reduce thyroid and other end-organ adverse effects and makes Dronedarone less lipophilic, with a shorter half-life. A placebo-controlled, double-blind, randomized, multicenter study to assess the effects of dronedarone 400 mg twice daily for 12 weeks on atrial fibrillation burden in subjects with permanent pacemakers.[Pubmed: 25638303]J Interv Card Electrophysiol. 2015 Mar;42(2):69-76. Dronedarone is a benzofuran derivative with a pharmacological profile similar to amiodarone but has a more rapid onset of action and a much shorter half-life (13-19 h). Our goal was to evaluate the efficacy of Dronedarone in atrial fibrillation (AF) patients using dual-chamber pacemakers capable of quantifying atrial fibrillation burden. |
| Animal Research | Dronedarone and digitalis: individually reduced post-repolarization refractoriness enhances life-threatening arrhythmias.[Pubmed: 25713011 ]Hepatic toxicity of dronedarone in mice: role of mitochondrial β-oxidation.[Pubmed: 24881592]Toxicology. 2014 Sep 2;323:1-9.Dronedarone is an amiodarone-like antiarrhythmic drug associated with severe liver injury. Since Dronedarone inhibits mitochondrial respiration and β-oxidation in vitro, mitochondrial toxicity may also explain Dronedarone-associated hepatotoxicity in vivo. Europace. 2015 Feb 20. pii: euu393. Interaction between Dronedarone and digitalis has been discussed as a possible cause for increased mortality in the presence of Dronedarone observed in the PALLAS trial. The aim of this study was to assess possible proarrhythmic effects of Dronedarone in combination with digitalis in an experimental whole heart model. |

Dronedarone Dilution Calculator

Dronedarone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7961 mL | 8.9805 mL | 17.9611 mL | 35.9221 mL | 44.9027 mL |
| 5 mM | 0.3592 mL | 1.7961 mL | 3.5922 mL | 7.1844 mL | 8.9805 mL |
| 10 mM | 0.1796 mL | 0.8981 mL | 1.7961 mL | 3.5922 mL | 4.4903 mL |
| 50 mM | 0.0359 mL | 0.1796 mL | 0.3592 mL | 0.7184 mL | 0.8981 mL |
| 100 mM | 0.018 mL | 0.0898 mL | 0.1796 mL | 0.3592 mL | 0.449 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Dronedarone(Multaq ) is a drug used for the treatment of atrial fibrillation and atrial flutter in patients who have suffered cardiac arrhythmias.
- (±)-BI-D
Catalog No.:BCC5537
CAS No.:1416258-16-6
- Dronedarone HCl
Catalog No.:BCC4777
CAS No.:141625-93-6
- Beta-D-glucopyranosyl oleanolate
Catalog No.:BCN6530
CAS No.:14162-53-9
- JW 642
Catalog No.:BCC6324
CAS No.:1416133-89-5
- UNC1215
Catalog No.:BCC2023
CAS No.:1415800-43-9
- Angustin B
Catalog No.:BCN7652
CAS No.:1415795-51-5
- Angustin A
Catalog No.:BCN7651
CAS No.:1415795-50-4
- ST-836 hydrochloride
Catalog No.:BCC1969
CAS No.:1415564-68-9
- PF-543 Citrate
Catalog No.:BCC1855
CAS No.:1415562-83-2
- PF-543
Catalog No.:BCC1854
CAS No.:1415562-82-1
- Crizotinib hydrochloride
Catalog No.:BCC5306
CAS No.:1415560-69-8
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- CU CPT 22
Catalog No.:BCC6320
CAS No.:1416324-85-0
- GR 94800
Catalog No.:BCC5799
CAS No.:141636-65-9
- Ivangustin
Catalog No.:BCN3507
CAS No.:14164-59-1
- Thrombin Receptor Activator for Peptide 5 (TRAP-5)
Catalog No.:BCC1025
CAS No.:141685-53-2
- NPEC-caged-D-AP5
Catalog No.:BCC7895
CAS No.:1416943-27-5
- Galanin (2-29) (rat)
Catalog No.:BCC5763
CAS No.:141696-11-9
- Faropenem daloxate
Catalog No.:BCC1571
CAS No.:141702-36-5
- RI-2
Catalog No.:BCC6424
CAS No.:1417162-36-7
- H-Phe(4-Cl)-OH
Catalog No.:BCC3171
CAS No.:14173-39-8
- H-DL-Phe(4-Cl)-OMe.HCl
Catalog No.:BCC3175
CAS No.:14173-40-1
- Exenatide acetate
Catalog No.:BCN8514
CAS No.:141732-76-5
- MM-102
Catalog No.:BCC4551
CAS No.:1417329-24-8
A placebo-controlled, double-blind, randomized, multicenter study to assess the effects of dronedarone 400 mg twice daily for 12 weeks on atrial fibrillation burden in subjects with permanent pacemakers.[Pubmed:25638303]
J Interv Card Electrophysiol. 2015 Mar;42(2):69-76.
PURPOSE: Dronedarone is a benzofuran derivative with a pharmacological profile similar to amiodarone but has a more rapid onset of action and a much shorter half-life (13-19 h). Our goal was to evaluate the efficacy of Dronedarone in atrial fibrillation (AF) patients using dual-chamber pacemakers capable of quantifying atrial fibrillation burden. METHODS: Pacemakers were adjusted to optimize AF detection. Patients with AF burden >1% were randomized to Dronedarone 400 mg twice daily (BID) or placebo. Pacemakers were interrogated after 4 and 12 weeks of treatment. The primary endpoint was the change in AF burden from baseline over the 12-week treatment period. Patients with permanent AF, severe/recently decompensated heart failure, and current use of antiarrhythmic drugs were excluded. AF burden was assessed by a core laboratory blinded to treatment assignment. RESULTS: From 285 patients screened, 112 were randomized (mean age 76 years, 60% male, 84% hypertensive, 65% with sick sinus syndrome, 26% with diabetes mellitus type II, 15% with heart failure). Baseline mean (SEM) AF burden was 8.77% (0.16) for placebo and 10.14% (0.17) for Dronedarone. Over the 12-week study period, AF burden compared to baseline decreased by 54.4% (0.22) (P = 0.0009) with Dronedarone and trended higher by 12.8% (0.16) (P = 0.450) with placebo. The absolute change in burden was decreased by 5.5% in the Dronedarone group and increased by 1.1% in the placebo group. Heart rate during AF was reduced to approximately 4 beats/min with Dronedarone (P = 0.285). Adverse events were higher with Dronedarone compared to placebo (65 vs 56%). CONCLUSIONS: Dronedarone reduced pacemaker-assessed the relative AF burden compared to baseline and placebo by over 50% during the 12-week observation period.
Hepatic toxicity of dronedarone in mice: role of mitochondrial beta-oxidation.[Pubmed:24881592]
Toxicology. 2014 Sep 2;323:1-9.
Dronedarone is an amiodarone-like antiarrhythmic drug associated with severe liver injury. Since Dronedarone inhibits mitochondrial respiration and beta-oxidation in vitro, mitochondrial toxicity may also explain Dronedarone-associated hepatotoxicity in vivo. We therefore studied hepatotoxicity of Dronedarone (200mg/kg/day for 2 weeks or 400mg/kg/day for 1 week by intragastric gavage) in heterozygous juvenile visceral steatosis (jvs(+/-)) and wild-type mice. Jvs(+/-) mice have reduced carnitine stores and are sensitive for mitochondrial beta-oxidation inhibitors. Treatment with Dronedarone 200mg/kg/day had no effect on body weight, serum transaminases and bilirubin, and hepatic mitochondrial function in both wild-type and jvs(+/-) mice. In contrast, Dronedarone 400mg/kg/day was associated with a 10-15% drop in body weight, and a 3-5-fold increase in transaminases and bilirubin in wild-type mice and, more accentuated, in jvs(+/-) mice. In vivo metabolism of intraperitoneal (14)C-palmitate was impaired in wild-type, and, more accentuated, in jvs(+/-) mice treated with 400mg/kg/day Dronedarone compared to vehicle-treated mice. Impaired beta-oxidation was also found in isolated mitochondria ex vivo. A likely explanation for these findings was a reduced activity of carnitine palmitoyltransferase 1a in liver mitochondria from Dronedarone-treated mice. In contrast, Dronedarone did not affect the activity of the respiratory chain ex vivo. We conclude that Dronedarone inhibits mitochondrial beta-oxidation in and ex vivo, but not the respiratory chain. Jvs(+/-) mice are slightly more sensitive for the effect of Dronedarone on mitochondrial beta-oxidation than wild-type mice. The results suggest that inhibition of mitochondrial beta-oxidation is an important mechanism of hepatotoxicity associated with Dronedarone.
The role of dronedarone in the treatment of atrial fibrillation/flutter in the aftermath of PALLAS.[Pubmed:24821656]
Curr Cardiol Rev. 2014 Nov;10(4):303-8.
Dronedarone is an amiodarone analog that differs structurally from amiodarone in that the iodine moiety was removed and a methane-sulfonyl group was added. These modifications reduce thyroid and other end-organ adverse effects and makes Dronedarone less lipophilic, with a shorter half-life. Dronedarone has been shown to prevent atrial fibrillation/ flutter (AF/AFl) recurrences in several multi-center trials. In addition to its rhythm control properties, Dronedarone has rate control properties. In patients with decompensated heart failure, Dronedarone treatment increased mortality and cardiovascular hospitalizations. When Dronedarone was used in elderly high risk AF/AFl patients, excluding those with advanced heart failure, cardiovascular hospitalizations were significantly reduced. The results of the PALLAS trial suggest that Dronedarone should not be used in the long-term treatment of patients with permanent AF. Post-marketing data have demonstrated rare hepatic toxicity to be associated with Dronedarone use. Updated practice and regulatory guidelines have positioned Dronedarone as a front-line antiarrhythmic in many patients with AF/Fl. However, the drug should not be used in patients with advanced heart failure and in patients who develop permanent AF.
Dronedarone and digitalis: individually reduced post-repolarization refractoriness enhances life-threatening arrhythmias.[Pubmed:25713011]
Europace. 2015 Aug;17(8):1300-8.
AIMS: Interaction between Dronedarone and digitalis has been discussed as a possible cause for increased mortality in the presence of Dronedarone observed in the PALLAS trial. The aim of this study was to assess possible proarrhythmic effects of Dronedarone in combination with digitalis in an experimental whole heart model. METHODS AND RESULTS: Twenty-six female rabbits underwent chronic oral treatment with Dronedarone (50 mg/kg/day for 6 weeks). Twenty-four rabbits received placebo. Heart failure was induced by rapid ventricular pacing. Sham-operated rabbits received a right-ventricular pacing lead but were not paced. Thereafter, hearts were isolated and Langendorff-perfused. Monophasic action potentials and a 12 lead electrocardiogram showed a dose-dependent decrease of QT interval, APD90, effective refractory periods, and postrepolarization refractoriness in control hearts and Dronedarone-pretreated hearts after application of ouabain (0.1 and 0.2 microM). After acute application of ouabain, ventricular fibrillation (VF) was inducible by programmed ventricular stimulation in 6 of 12 untreated sham hearts (38 episodes) as compared with 7 of 11 Dronedarone-pretreated sham hearts (76 episodes). In untreated failing hearts, 6 of 12 hearts were inducible (47 episodes) as compared with 7 of 15 hearts Dronedarone-pretreated failing hearts (93 episodes). CONCLUSION: In this study, ouabain treatment resulted in an increased ventricular vulnerability in chronically Dronedarone-pretreated control and failing hearts. Ouabain led to a significant abbreviation of ventricular repolarization. This was more marked in Dronedarone-pretreated hearts and resulted in an elevated incidence of VF. This may help to interpret the results of the PALLAS trial.
Dronedarone and renal impairment: evaluation of Spanish postmarketing reports and review of literature.[Pubmed:25967281]
Expert Opin Drug Saf. 2015 Jun;14(6):807-13.
BACKGROUND: Renal impairment associated with Dronedarone use is hardly known. Our aim is to describe the characteristics of spontaneous reports involving renal adverse reactions with use of Dronedarone. METHODS: In the Spanish Pharmacovigilance database, reports with renal reactions and Dronedarone until May 2014 were retrieved and analyzed. Also, a review of case reports of renal failure and Dronedarone was conducted in MEDLINE. RESULTS: Dronedarone was found as a suspected drug in 192 reports, 10 (5.2%) of these reports described renal reactions. Renal reactions appeared until 3 months after the onset of Dronedarone treatment. In 5 out of 10 cases, Dronedarone was withdrawn and the patient recovered. The Reporting Odds Ratio was 2.88 [95% CI 1.52 - 5.46; p < 0.05]. Additionally, eight cases were found in the medical literature. In five of them, the patient outcome was described as recovered. One patient had to undergo hemodialysis for the treatment of their renal impairment. CONCLUSIONS: The effect of Dronedarone on the renal function is supported by limited information; therefore, the cases from spontaneous reporting system and those from the medical literature could give relevant additional information. Our analysis shows a potential relationship between Dronedarone use and renal impairment. Further studies are needed to confirm these findings.


