JW 642MAGL inhibitor CAS# 1416133-89-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1416133-89-5 | SDF | Download SDF |
| PubChem ID | 71656520 | Appearance | Powder |
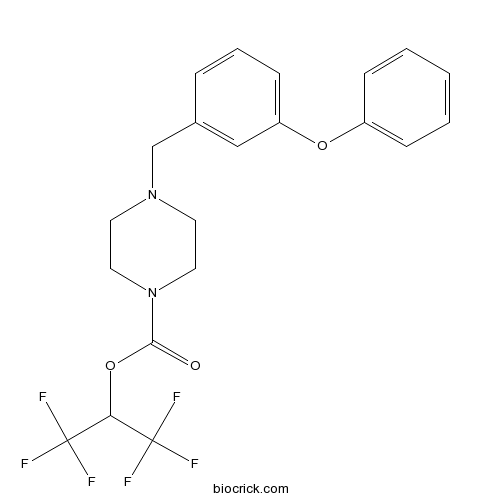
| Formula | C21H20F6N2O3 | M.Wt | 462.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (216.27 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 1,1,1,3,3,3-hexafluoropropan-2-yl 4-[(3-phenoxyphenyl)methyl]piperazine-1-carboxylate | ||
| SMILES | C1CN(CCN1CC2=CC(=CC=C2)OC3=CC=CC=C3)C(=O)OC(C(F)(F)F)C(F)(F)F | ||
| Standard InChIKey | AVSCNEOUWSVZEY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H20F6N2O3/c22-20(23,24)18(21(25,26)27)32-19(30)29-11-9-28(10-12-29)14-15-5-4-8-17(13-15)31-16-6-2-1-3-7-16/h1-8,13,18H,9-12,14H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective monoacylglycerol lipase (MAGL) inhibitor (IC50 = 3.7 nM). Displays >1000-fold selectivity for MAGL over fatty acid amide hydrolase (IC50 = 20.6 μM). Analog of JZL 195. |

JW 642 Dilution Calculator

JW 642 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1627 mL | 10.8134 mL | 21.6268 mL | 43.2535 mL | 54.0669 mL |
| 5 mM | 0.4325 mL | 2.1627 mL | 4.3254 mL | 8.6507 mL | 10.8134 mL |
| 10 mM | 0.2163 mL | 1.0813 mL | 2.1627 mL | 4.3254 mL | 5.4067 mL |
| 50 mM | 0.0433 mL | 0.2163 mL | 0.4325 mL | 0.8651 mL | 1.0813 mL |
| 100 mM | 0.0216 mL | 0.1081 mL | 0.2163 mL | 0.4325 mL | 0.5407 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
JW 642 is a potent inhibitor of monoacylglycerol lipase (MAGL) that displays IC50 values of 7.6, 14, and 3.7 nM for inhibition of MAGL in mouse, rat, and human brain membranes, respectively. IC50 value: 7.6/14/3.7 nM(mouse/rat/human MAGL) [1] Target: MAGL inhibitor JW 642 is selective for MAGL, requiring much higher concentrations to effectively inhibit fatty acid amide hydrolase activity (IC50s = 31, 14, and 20.6 μM for mouse, rat, and human brain membranes, respectively).
References:
[1]. Chang JW, et al. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol. 2012 May 25;19(5):579-88.
- UNC1215
Catalog No.:BCC2023
CAS No.:1415800-43-9
- Angustin B
Catalog No.:BCN7652
CAS No.:1415795-51-5
- Angustin A
Catalog No.:BCN7651
CAS No.:1415795-50-4
- ST-836 hydrochloride
Catalog No.:BCC1969
CAS No.:1415564-68-9
- PF-543 Citrate
Catalog No.:BCC1855
CAS No.:1415562-83-2
- PF-543
Catalog No.:BCC1854
CAS No.:1415562-82-1
- Crizotinib hydrochloride
Catalog No.:BCC5306
CAS No.:1415560-69-8
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- CDK9 inhibitor
Catalog No.:BCC1465
CAS No.:1415559-43-1
- MK-8245 Trifluoroacetate
Catalog No.:BCC1769
CAS No.:1415559-41-9
- LY 231617
Catalog No.:BCC7005
CAS No.:141545-89-3
- Pelandjauic acid
Catalog No.:BCN3752
CAS No.:141545-69-9
- Beta-D-glucopyranosyl oleanolate
Catalog No.:BCN6530
CAS No.:14162-53-9
- Dronedarone HCl
Catalog No.:BCC4777
CAS No.:141625-93-6
- (±)-BI-D
Catalog No.:BCC5537
CAS No.:1416258-16-6
- Dronedarone
Catalog No.:BCN2176
CAS No.:141626-36-0
- CU CPT 22
Catalog No.:BCC6320
CAS No.:1416324-85-0
- GR 94800
Catalog No.:BCC5799
CAS No.:141636-65-9
- Ivangustin
Catalog No.:BCN3507
CAS No.:14164-59-1
- Thrombin Receptor Activator for Peptide 5 (TRAP-5)
Catalog No.:BCC1025
CAS No.:141685-53-2
- NPEC-caged-D-AP5
Catalog No.:BCC7895
CAS No.:1416943-27-5
- Galanin (2-29) (rat)
Catalog No.:BCC5763
CAS No.:141696-11-9
- Faropenem daloxate
Catalog No.:BCC1571
CAS No.:141702-36-5
- RI-2
Catalog No.:BCC6424
CAS No.:1417162-36-7
Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates.[Pubmed:22542104]
Chem Biol. 2012 May 25;19(5):579-88.
The endocannabinoids 2-arachidonoyl glycerol (2-AG) and N-arachidonoyl ethanolamine (anandamide) are principally degraded by monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH), respectively. The recent discovery of O-aryl carbamates such as JZL184 as selective MAGL inhibitors has enabled functional investigation of 2-AG signaling pathways in vivo. Nonetheless, JZL184 and other reported MAGL inhibitors still display low-level cross-reactivity with FAAH and peripheral carboxylesterases, which can complicate their use in certain biological studies. Here, we report a distinct class of O-hexafluoroisopropyl (HFIP) carbamates that inhibits MAGL in vitro and in vivo with excellent potency and greatly improved selectivity, including showing no detectable cross-reactivity with FAAH. These findings designate HFIP carbamates as a versatile chemotype for inhibiting MAGL and should encourage the pursuit of other serine hydrolase inhibitors that bear reactive groups resembling the structures of natural substrates.


