LY 231617Antioxidant; neuroprotective in vitro and in vivo CAS# 141545-89-3 |
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- GW3965
Catalog No.:BCC1612
CAS No.:405911-09-3
- GW3965 HCl
Catalog No.:BCC3790
CAS No.:405911-17-3
- Fexaramine
Catalog No.:BCC7412
CAS No.:574013-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 141545-89-3 | SDF | Download SDF |
| PubChem ID | 178576 | Appearance | Powder |
| Formula | C17H30ClNO | M.Wt | 299.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
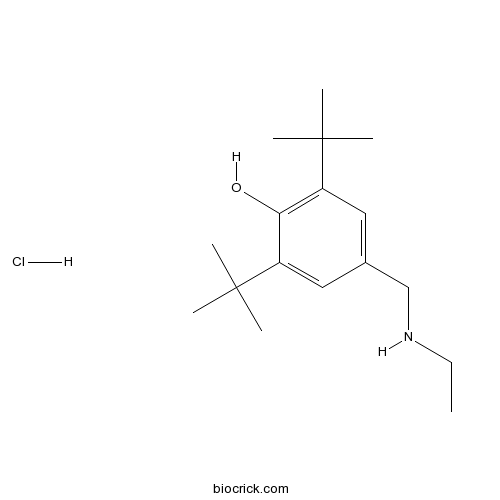
| Chemical Name | 2,6-ditert-butyl-4-(ethylaminomethyl)phenol;hydrochloride | ||
| SMILES | CCNCC1=CC(=C(C(=C1)C(C)(C)C)O)C(C)(C)C.Cl | ||
| Standard InChIKey | SIWZKGFBUXQFDK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H29NO.ClH/c1-8-18-11-12-9-13(16(2,3)4)15(19)14(10-12)17(5,6)7;/h9-10,18-19H,8,11H2,1-7H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antioxidant; protects against ischemia-induced neuronal damage in rat models of global and focal cerebral ischemia. Neuroprotective in vitro and in vivo. |

LY 231617 Dilution Calculator

LY 231617 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3347 mL | 16.6733 mL | 33.3467 mL | 66.6933 mL | 83.3667 mL |
| 5 mM | 0.6669 mL | 3.3347 mL | 6.6693 mL | 13.3387 mL | 16.6733 mL |
| 10 mM | 0.3335 mL | 1.6673 mL | 3.3347 mL | 6.6693 mL | 8.3367 mL |
| 50 mM | 0.0667 mL | 0.3335 mL | 0.6669 mL | 1.3339 mL | 1.6673 mL |
| 100 mM | 0.0333 mL | 0.1667 mL | 0.3335 mL | 0.6669 mL | 0.8337 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pelandjauic acid
Catalog No.:BCN3752
CAS No.:141545-69-9
- 5S rRNA modificator
Catalog No.:BCC5442
CAS No.:1415238-77-5
- Levosimendan
Catalog No.:BCC4793
CAS No.:141505-33-1
- Aloin A
Catalog No.:BCN1042
CAS No.:1415-73-2
- Rauvoyunine B
Catalog No.:BCN6995
CAS No.:1414883-82-1
- Rauvoyunine A
Catalog No.:BCN7002
CAS No.:1414883-81-0
- Guajadial B
Catalog No.:BCN3972
CAS No.:1414455-03-0
- Diosgenin glucoside
Catalog No.:BCN1250
CAS No.:14144-06-0
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- MRS 2219
Catalog No.:BCC6966
CAS No.:14141-47-0
- PX 12
Catalog No.:BCC2436
CAS No.:141400-58-0
- Trachelanthamine
Catalog No.:BCN2041
CAS No.:14140-18-2
- MK-8245 Trifluoroacetate
Catalog No.:BCC1769
CAS No.:1415559-41-9
- CDK9 inhibitor
Catalog No.:BCC1465
CAS No.:1415559-43-1
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- Crizotinib hydrochloride
Catalog No.:BCC5306
CAS No.:1415560-69-8
- PF-543
Catalog No.:BCC1854
CAS No.:1415562-82-1
- PF-543 Citrate
Catalog No.:BCC1855
CAS No.:1415562-83-2
- ST-836 hydrochloride
Catalog No.:BCC1969
CAS No.:1415564-68-9
- Angustin A
Catalog No.:BCN7651
CAS No.:1415795-50-4
- Angustin B
Catalog No.:BCN7652
CAS No.:1415795-51-5
- UNC1215
Catalog No.:BCC2023
CAS No.:1415800-43-9
- JW 642
Catalog No.:BCC6324
CAS No.:1416133-89-5
- Beta-D-glucopyranosyl oleanolate
Catalog No.:BCN6530
CAS No.:14162-53-9
The antioxidant LY 231617 ameliorates functional and morphological sequelae induced by global ischemia in rats.[Pubmed:8974659]
Brain Res. 1995 Oct 2;694(1-2):308-11.
In this study the effect of LY 231617, an antioxidant, on spatial learning deficit and on neuronal damage following transient cerebral ischemia was evaluated. Global ischemia was induced by four-vessel-occlusion (4VO) for 20 min in rats. LY 231617 (20 mg/kg i.p.) was administered after onset of reperfusion. One week after surgery spatial learning was tested in the Morris water maze. LY 231617 reduced the increase in escape latency and in swim distance induced by 4VO. Neuronal damage in the CAI sector of the hippocampus produced by 4VO was significantly attenuated by LY 231617. The present data demonstrate that posttreatment with LY 231617 exerts a protective effect on hippocampal neuronal damage and deficits in spatial learning induced by 4VO.
Characterization of LY231617 protection against hydrogen peroxide toxicity.[Pubmed:10037488]
J Neurochem. 1999 Mar;72(3):1154-60.
The compound LY231617 [2,6-bis(1,1-dimethylethyl)-4-[[(1-ethyl)amino]methyl]phenol hydrochloride] has been reported to afford significant neuroprotection against hydrogen peroxide (H2O2)-induced toxicity in vitro and global ischemia in vivo. We now report on further mechanistic studies of H2O2 toxicity and protection by LY231617. Brief exposure to H2O2 (15 min) elicited an oxidative insult comparable with that generated by overnight treatment. H2O2-mediated cellular degeneration was characterized using lactate dehydrogenase (LDH) release, changes in total glutathione, and a new marker of oxidative stress, 8-epiprostaglandin F2alpha (8-isoprostane). LY231617 attenuated H2O2-mediated degeneration under a variety of exposure conditions, including a more clinically relevant posttreatment paradigm. Levels of 8-isoprostane paralleled LDH release under various treatment paradigms of 100 microM H2O2 +/- 5 microM drug. In contrast, despite affording significant protection, LY231617 had modest to no effects on cellular levels of glutathione. Taken together, these results are consistent with a membrane site of action for LY231617 and suggest that the compound affords cytoprotection via its antioxidant properties.
Neuroprotective effects of the antioxidant LY231617 and NO synthase inhibitors in global cerebral ischaemia.[Pubmed:9237532]
Brain Res. 1997 Jun 20;760(1-2):170-8.
Recent studies have shown that the novel antioxidant LY231617 protects against ischaemia-induced neuronal damage in rat models of global cerebral ischaemia. In the present studies we have examined the effects of LY231617 in the gerbil model of global cerebral ischaemia. We also examined the effects of four nitric oxide synthase inhibitors (3-bromo-7-nitroindazole, N(G)-nitro-L-arginine methyl ester, aminoguanidine and S-methylisothiourea sulphate) in this model. LY231617 (50 mg/kg p.o. or 30 mg/kg i.p.) was administered either 30 min prior to occlusion or immediately post-occlusion followed by three further doses at 4, 24 and 48 h after the initial dose. 3-Bromo-7-nitroindazole was administered at 40 mg/kg i.p. immediately after occlusion followed by 20 mg/kg i.p. at 3, 6, 24 and 48 h, N(G)-nitro-L-arginine methyl ester was administered at 10 mg/kg i.p. immediately after occlusion followed by 5 mg/kg i.p. at 3, 6, 24 and 48 h. Aminoguanidine was administered at 80 mg/kg i.p. immediately after occlusion followed by 40 mg/kg i.p. at 3, 6, 24 and 48 h and S-methylisothiourea sulphate was administered at 10 mg/kg i.p. immediately, 3, 6, 24 and 48 h after occlusion. We also examined the effects of aminoguanidine administered at 80 mg/kg i.p. immediately after occlusion followed by 40 mg/kg i.p. at 3, 6, 24, 48, 72 and 96 h and S-methylisothiourea sulphate administered at 10 mg/kg i.p. immediately, 3, 6, 24, 48, 72 and 96 h after occlusion. Control animals were either sham operated or subjected to 5 min bilateral carotid occlusion. Extensive neuronal death was observed in the CA1 layer of the hippocampus in 5-min bilateral carotid artery occluded animals 5 days after surgery. LY231617 provided significant neuroprotection against the ischaemia-induced brain damage when administration was initiated before or after occlusion (P < 0.05). The neuronal NO synthase inhibitors, 3-bromo-7-nitroindazole and a general NO synthase inhibitor, N(G)-nitro-L-arginine methyl ester also provided significant neuroprotection (P < 0.05). In contrast aminoguanidine and S-methylisothiourea sulphate (two inducible NO synthase inhibitors) failed to protect against the ischaemia-induced brain damage. These results indicate that free radicals and nitric oxide are involved in ischaemia-induced brain damage following global cerebral ischaemia. Antioxidants such as LY231617 or neuronal NO synthase inhibitors can prevent the ischaemia-induced neurodegeneration and may be useful as anti-ischaemic agents.
The antioxidant LY231617 reduces global ischemic neuronal injury in rats.[Pubmed:8488528]
Stroke. 1993 May;24(5):716-22; discussion 722-3.
BACKGROUND AND PURPOSE: In the rat four-vessel occlusion model with 30 minutes of ischemia most agents have failed to be of benefit when given after ischemia. Because postischemia administration is more clinically relevant, we evaluated the antioxidant LY231617 (2,6-bis(1,1-dimethylethyl)-4-[[(1-ethyl)amino]methyl]phenol hydrochloride]) when administered after 30 minutes of four-vessel occlusion. METHODS: Male Wistar rats were subjected to 30 minutes of four-vessel occlusion. LY231617 was either given orally 30 minutes before ischemia or intravenously beginning at 30 minutes after the onset of ischemia. Hippocampal CA1 layer and striatal damage were rated on a scale of 0-3 (0, no damage; 3, > 90% cell loss). We also evaluated the ability of LY231617 to prevent iron-dependent lipid peroxidation and to prevent hydrogen peroxide-induced neuronal death of hippocampal neurons in primary culture by exposing cultures to a 50-microM concentration of hydrogen peroxide for 15 minutes in the presence of LY231617. RESULTS: Oral administration of LY231617 reduced both striatal and hippocampal CA1 damage by > 75% (p < 0.0001). In two separate experiments in which LY231617 was given intravenously beginning 30 minutes after occlusion, hippocampal and striatal damage were reduced by approximately 50% (p < 0.03) in the first experiment and by approximately 41% (p < 0.02) in the second experiment. Addition of 5 microM of LY231617 to primary hippocampal neuronal cultures antagonized the lethal effect of hydrogen peroxide (p < 0.05). Iron-dependent lipid peroxidation was also inhibited in a dose-related fashion. CONCLUSIONS: The significant reduction of ischemia-induced or hydrogen peroxide-induced neuronal damage and inhibition of lipid peroxidation by LY231617 observed in this study suggest that reactive oxygen intermediates play an important role in the events leading to neuronal death after global ischemia/reperfusion.


