PX 12Trx-1 inhibitor CAS# 141400-58-0 |
- BS-181
Catalog No.:BCC1439
CAS No.:1092443-52-1
- CDK inhibitor II
Catalog No.:BCC1464
CAS No.:1269815-17-9
- CGP60474
Catalog No.:BCC1474
CAS No.:164658-13-3
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- AT7519
Catalog No.:BCC2541
CAS No.:844442-38-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 141400-58-0 | SDF | Download SDF |
| PubChem ID | 219104 | Appearance | Powder |
| Formula | C7H12N2S2 | M.Wt | 188.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | IV-2 | ||
| Solubility | Ethanol : 50 mg/mL (265.52 mM; Need ultrasonic) DMSO : ≥ 44.7 mg/mL (237.37 mM) *"≥" means soluble, but saturation unknown. | ||
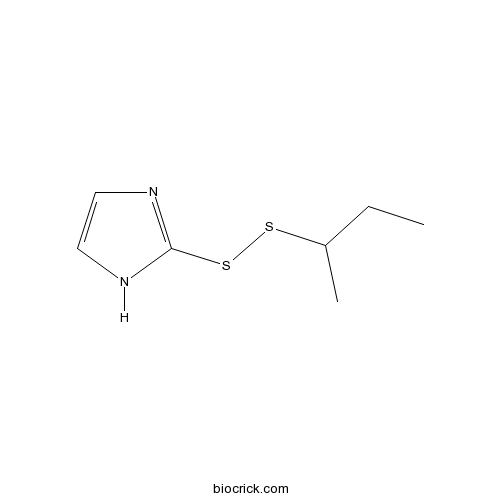
| Chemical Name | 2-(butan-2-yldisulfanyl)-1H-imidazole | ||
| SMILES | CCC(C)SSC1=NC=CN1 | ||
| Standard InChIKey | BPBPYQWMFCTCNG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H12N2S2/c1-3-6(2)10-11-7-8-4-5-9-7/h4-6H,3H2,1-2H3,(H,8,9) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Thioredoxin-1 (Trx-1) inhibitor; irreversibly inactivates Trx-1 as a substrate for reduction by thioredoxin reductase (TR); competitively inhibits the reduction of Trx-1 by TR (Ki = 30.8 μM). Attenuates expression of HIF-1α, VEGF and iNOS (IC50 values are 7.2, 10.4 and 18.1 μM respectively). Exhibits antitumor activity; directly inhibits tubulin in vitro and decreases tumor microvessel density in vivo. |

PX 12 Dilution Calculator

PX 12 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.3104 mL | 26.552 mL | 53.1039 mL | 106.2078 mL | 132.7598 mL |
| 5 mM | 1.0621 mL | 5.3104 mL | 10.6208 mL | 21.2416 mL | 26.552 mL |
| 10 mM | 0.531 mL | 2.6552 mL | 5.3104 mL | 10.6208 mL | 13.276 mL |
| 50 mM | 0.1062 mL | 0.531 mL | 1.0621 mL | 2.1242 mL | 2.6552 mL |
| 100 mM | 0.0531 mL | 0.2655 mL | 0.531 mL | 1.0621 mL | 1.3276 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PX 12 is an inhibitor of thioredoxin-1 [1].
Thioredoxin-1 (Trx-1) is a small redox protein with a conserved catalytic site and plays an important role in cells that includes the regulation of trans-activating activity and the DNA binding of redox-sensitive transcription factors [1].
In HT-29 human colon carcinoma cells and MCF-7 human breast cancer, PX 12 prevented the hypoxia-induced increase in HIF-1 protein. Also, PX 12 decreased inducible nitric oxide synthase, HIF-1-trans-activating activity and VEGF formation [2].
In immunodeficient mice bearing HT-29 human colon xenografts, PX 12 decreased the average tumor blood vessel permeability by 63% within 2 hours and returned to pretreatment values after 48 hours. PX 12 reduced tumor-derived VEGF and tumor after 24 hours. Also, Trx-1 showed a rapid decrease within 2 hours and maintained for 24 hours [1]. In mice bearing MCF-7 tumor xenografts, PX 12 reduced HIF-1ɑ and VEGF protein levels [2]. In cancer patients, PX-12 treatment significantly reduced the levels of Trx-1 and VEGF in plasma [3].
References:
[1]. Jordan BF, Runquist M, Raghunand N, et al. The thioredoxin-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) decreases vascular permeability in tumor xenografts monitored by dynamic contrast enhanced magnetic resonance imaging. Clin Cancer Res, 2005, 11(2 Pt 1): 529-536.
[2]. Welsh SJ, Williams RR, Birmingham A, et al. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther, 2003, 2(3): 235-243.
[3]. Baker AF, Dragovich T, Tate WR, et al. The antitumor thioredoxin-1 inhibitor PX-12 (1-methylpropyl 2-imidazolyl disulfide) decreases thioredoxin-1 and VEGF levels in cancer patient plasma. J Lab Clin Med, 2006, 147(2): 83-90.
- Trachelanthamine
Catalog No.:BCN2041
CAS No.:14140-18-2
- Orobanchyl acetate
Catalog No.:BCN7779
CAS No.:1413843-71-6
- PEPA
Catalog No.:BCC5951
CAS No.:141286-78-4
- QS-21
Catalog No.:BCC8243
CAS No.:141256-04-4
- Asenapine hydrochloride
Catalog No.:BCC1371
CAS No.:1412458-61-7
- Btk inhibitor 1
Catalog No.:BCC4238
CAS No.:1412418-47-3
- 2-(Dimethylaminomethyl)-2-propanol
Catalog No.:BCN1774
CAS No.:14123-48-9
- Epiguajadial B
Catalog No.:BCN4075
CAS No.:1411629-26-9
- TRAP-6
Catalog No.:BCC3957
CAS No.:141136-83-6
- 12-Hydroxydodecanoic Acid
Catalog No.:BCN8405
CAS No.:505-95-3
- JNK-IN-8
Catalog No.:BCC1673
CAS No.:1410880-22-6
- Xanomeline oxalate
Catalog No.:BCC4146
CAS No.:141064-23-5
- MRS 2219
Catalog No.:BCC6966
CAS No.:14141-47-0
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- Diosgenin glucoside
Catalog No.:BCN1250
CAS No.:14144-06-0
- Guajadial B
Catalog No.:BCN3972
CAS No.:1414455-03-0
- Rauvoyunine A
Catalog No.:BCN7002
CAS No.:1414883-81-0
- Rauvoyunine B
Catalog No.:BCN6995
CAS No.:1414883-82-1
- Aloin A
Catalog No.:BCN1042
CAS No.:1415-73-2
- Levosimendan
Catalog No.:BCC4793
CAS No.:141505-33-1
- 5S rRNA modificator
Catalog No.:BCC5442
CAS No.:1415238-77-5
- Pelandjauic acid
Catalog No.:BCN3752
CAS No.:141545-69-9
- LY 231617
Catalog No.:BCC7005
CAS No.:141545-89-3
- MK-8245 Trifluoroacetate
Catalog No.:BCC1769
CAS No.:1415559-41-9
Upregulation of connexin43 contributes to PX-12-induced oxidative cell death.[Pubmed:26684802]
Tumour Biol. 2016 Jun;37(6):7535-46.
Thioredoxin (Trx) is a small redox protein that underlies aggressive tumor growth and resistance to chemotherapy. Inhibition of Trx with the chemical inhibitor PX-12 suppresses tumor growth and induces cell apoptosis. Currently, the mechanism underlying the therapeutic actions of PX-12 and the molecules influencing cell susceptibility to PX-12 are incompletely understood. Given that connexin43 (Cx43), a tumor suppressor, regulates tumor cell susceptibility to chemotherapy, we examined the possible involvement of Cx43 in PX-12-induced cell death. Exposure of cells to PX-12 led to a loss of cell viability, which was associated with the activation of oxidative sensitive c-Jun N-terminal kinase (JNK). Inhibition of JNK or supplement of cells with anti-oxidants prevented the cell-killing action of PX-12. The forced expression of Cx43 in normal and tumor cells increased cell sensitivity to PX-12-induced JNK activation and cell death. In contrast, the downregulation of Cx43 with siRNA or the suppression of gap junctions with chemical inhibitors attenuated JNK activation and enhanced cell resistance to PX-12. Further analysis revealed that PX-12 at low concentrations induced a JNK-dependent elevation in the Cx43 protein, which was also preventable by supplementing the cells with anti-oxidants. Our results thus indicate that Cx43 is a determinant in the regulation of cell susceptibility to PX-12 and that the upregulation of Cx43 may be an additional mechanism by which PX-12 exerts its anti-tumor actions.
Thioredoxin-1 inhibitor PX-12 induces human acute myeloid leukemia cell apoptosis and enhances the sensitivity of cells to arsenic trioxide.[Pubmed:25197347]
Int J Clin Exp Pathol. 2014 Jul 15;7(8):4765-73. eCollection 2014.
Thioredoxin-1 (Trx-1), an important redox regulatory factor, plays a significant role in drug-induced apoptosis. Here we investigated the effects of the Trx-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) on human acute myeloid leukemia cells (AML) and the sensitivity of cells to arsenic trioxide (As2O3, ATO). Treatment of cells with a different concentration of PX-12 for 48 h resulted in growth inhibition, the induction of apoptosis and increased the levels of activated caspase-3 expression in AML cell lines HL-60, NB4, U937 and primary AML cells in a dose-dependent manner. In addition, PX-12 enhanced the sensitivity of U937 cells to ATO. These results suggest the effects of Trx-1 inhibitor PX-12 to induce apoptosis in AML cells and therapeutic potential in AML by enhancing the sensitivity of cells to ATO.
PX-12 inhibits the growth of hepatocelluar carcinoma by inducing S-phase arrest, ROS-dependent apoptosis and enhances 5-FU cytotoxicity.[Pubmed:26550453]
Am J Transl Res. 2015 Sep 15;7(9):1528-40. eCollection 2015.
BACKGROUND: 1-methylpropyl 2-imidazolyl disulfide (PX-12), a thioredoxin 1 (Trx1) inhibitor, has been investigated in a number of ancers, but its effectiveness in the treatment of hepatocellular carcinoma (HCC) has not been reported. PX-12 has generated considerable interest in its use in a variety of solid tumors, yet most studies have confined their interests to using PX-12 as a single agent. The aim of this study is to investigate whether PX-12 inhibits cell growth and has a synergistic anti-tumor effect in combination with 5-fluorouracil (5-FU) in HCC. METHODS: Cells were treated with different concentrations of PX-12 and 5-FU. Cell viability assays, colony formation assay, cell cycle assay, reactive oxygen species (ROS) assay, apoptosis analysis, western blot assay, immunohistochemistry and xenograft tumorigenicity assay were performed. RESULTS: Treatment with PX-12 inhibited cell growth, induced S-phase arrest, and increased ROS levels. PX-12-induced apoptosis and inhibition of colony formation were associated with the generation of ROS, and inhibition of ROS attenuated PX-12-induced apoptosis and inhibition of colony formation. Treatment with PX-12 increased the expression of bax and reduced the expression of bcl-2, indicating that PX-12-mediated apoptosis is mitochondria-dependent. PX-12 also exerted a synergistic effect with 5-FU tosignificantly suppress tumorigenicity both in vitro and in vivo. Inhibition of ROS accumulation reduced the synergistic effect of PX-12 and 5-FU. CONCLUSIONS: PX-12 has anti-tumor activity and a synergistic effect in combination with 5-FU in HCC. Treatment with PX-12 alone or in combination with 5-FU may have clinical use in the treatment of HCC and other cancers.
PX-12 induces apoptosis in Calu-6 cells in an oxidative stress-dependent manner.[Pubmed:25391429]
Tumour Biol. 2015 Mar;36(3):2087-95.
PX-12 (1-methylpropyl 2-imidazolyl disulfide) as a thioredoxin (Trx) inhibitor has an anti-tumor effect. However, there is no report about the toxicological effect of PX-12 on lung cancer cells. Here, we investigated the anti-growth effects of PX-12 on Calu-6 lung cancer cells in relation to reactive oxygen species (ROS) and glutathione (GSH) levels. PX-12 induced the growth inhibition of Calu-6 cells with IC50 of nearly 3 muM at 72 h. In contrast, PX-12 did not affect the growth of human small airway epithelial cells (HSAECs). Cell cycle distribution analysis indicated that PX-12 significantly induced a G2/M phase arrest in Calu-6 cells. PX-12 also increased the number of annexin V-FITC-positive cells in Calu-6 cells. All the tested caspase inhibitors markedly prevented Calu-6 cell death induced by PX-12. With regard to ROS and GSH levels, PX-12 increased ROS levels containing O2(.-) in Calu-6 cells and induced the depletion of GSH. N-acetyl cysteine (NAC), which is a well-known antioxidant, significantly reduced O2(.-) level in PX-12-treated Calu-6 cells and prevented apoptosis and GSH depletion in these cells. In conclusion, it is the first report that PX-12 inhibited the growth of Calu-6 cells via a G2/M phase arrest as well as apoptosis, which effect was related to the intracellular increases in ROS levels.
The thioredoxin-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) decreases vascular permeability in tumor xenografts monitored by dynamic contrast enhanced magnetic resonance imaging.[Pubmed:15701837]
Clin Cancer Res. 2005 Jan 15;11(2 Pt 1):529-36.
PURPOSE: The purpose of this study was to use dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) to measure changes in tumor xenograft permeability produced by the antitumor thioredoxin-1 (Trx-1) inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) and to assess the relationship to Trx-1 and vascular endothelial growth factor (VEGF) levels. EXPERIMENTAL DESIGN: DCE-MRI was used to monitor the dynamics of gadolinium-diethylenetriaminepentaacetic acid coupled bovine serum albumin as a macromolecular contrast reagent to measure hemodynamic changes in HT-29 human colon xenografts in immunodeficient mice treated with PX-12. Blood vessel permeability was estimated from the slope of the enhancement curves, and tumor vascular volume fraction from the ordinate. Tumor Trx-1 and VEGF was also measured. RESULTS: PX-12 caused a rapid 63% decrease in the average tumor blood vessel permeability within 2 hours of administration. The decrease lasted 24 hours and had returned to pretreatment values by 48 hours. The changes in vascular permeability were not accompanied by alterations in average tumor vascular volume fraction. There was a decrease in tumor and tumor-derived VEGF in plasma at 24 hours after treatment with PX-12, but not at earlier time points. However, tumor redox active Trx-1 showed a rapid decline within 2 hours following PX-12 administration that was maintained for 24 hours. CONCLUSION: The rapid decrease in tumor vascular permeability caused by PX-12 administration coincided with a decrease in tumor redox active Trx-1 and preceded a decrease in VEGF. DCE-MRI responses to PX-12 in patients of Trx-1 inhibition at early time points and decreased VEGF at later times, may be useful to follow tumor response and even therapeutic benefit.
The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation.[Pubmed:12657718]
Mol Cancer Ther. 2003 Mar;2(3):235-43.
Hypoxia-inducible factor-1 (HIF-1) is a transcription factor that plays a critical role in tumor growth by increasing resistance to apoptosis and the production of angiogenic factors such as vascular endothelial growth factor (VEGF). HIF-1 is a heterodimer comprised of oxygen-regulated HIF-1alpha and constitutively expressed HIF-1beta subunits. The redox protein thioredoxin-1 (Trx-1), which is found at high levels in many human cancers, increases both aerobic and hypoxia-induced HIF-1alpha protein in cells leading to increased expression of HIF-regulated genes. We have investigated whether two cancer drugs that inhibit Trx-1 signaling, PX-12 (1-methylpropyl 2-imidazolyl disulfide) and pleurotin, decrease HIF-1alpha protein levels and the expression of downstream target genes. Treatment of MCF-7 human breast cancer and HT-29 human colon carcinoma cells with PX-12 and pleurotin prevented the hypoxia (1% oxygen)-induced increase in HIF-1alpha protein. HIF-1-trans-activating activity, VEGF formation, and inducible nitric oxide synthase were also decreased by treatment with PX-12 and pleurotin under hypoxic conditions. PX-12 and pleurotin also decreased HIF-1alpha protein levels and HIF-1 trans-activation in RCC4 renal cell carcinoma cells that constitutively overexpress HIF-1alpha protein because of loss of the pVHL gene, indicating that HIF-1alpha is inhibited independently of the pVHL pathway. HIF-1alpha and VEGF protein levels in MCF-7 tumor xenografts in vivo were decreased by PX-12 treatment of mice. The results suggest that inhibition of HIF-1alpha by Trx-1 inhibitors may contribute to the growth inhibitory and antitumor activity of these agents.
Mechanisms of inhibition of the thioredoxin growth factor system by antitumor 2-imidazolyl disulfides.[Pubmed:9605422]
Biochem Pharmacol. 1998 Apr 1;55(7):987-94.
The interactions of a series of 2-imidazolyl disulfide antitumor compounds with the thioredoxin reductase(TR)/thioredoxin (hTrx) redox system have been studied. Disulfides III-2 (n-butyl 2-mercaptoimidazolyl disulfide) and VI-2 (ethyl 2-mercaptoimidazolyl disulfide) were substrates for reduction by TR with Km values of 43 and 48 microM. Disulfides IV-2 (1-methylpropyl 2-mercaptoimidazolyl disulfide) and DLK-36 (benzyl 2-mercaptoimidazolyl disulfide) were competitive inhibitors of the reduction of hTrx by TR with Ki values of 31 microM. None of the disulfides were substrates for reduction by human glutathione reductase. The disulfides caused reversible thioalkylation of hTrx at the redox catalytic site as shown by the fact that there was no thioalkylation of a mutant hTrx where both the catalytic site Cys32 and Cys35 residues were replaced by Ser. In addition, the disulfides caused a slower irreversible inactivation of hTrx as a substrate for reduction by TR, with half-lives for III-2 of 30 min, for IV-2 of 4 hr, and for IX-2 (t-butyl 2-mercaptoimidazolyl disulfide) of 24 hr. This irreversible inactivation of hTrx occurred at concentrations of the disulfides an order of magnitude below those that inhibited TR, and involved the Cys73 of hTrx, which is outside the conserved redox catalytic site, as shown by the resistance to inactivation of a mutant hTrx where Cys73 was replaced by Ser. Electrophoretic and mass spectral analyses of the products of the reaction between the disulfides and hTrx show that modification of 1-3 Cys residues of the protein occurred in a concentration-dependent fashion. The disulfides inhibited the hTrx-dependent proliferation of MCF-7 breast cancer cells with IC50 values for III-2 and IV-2 of 0.2 and 1.2 microM, respectively. The results show that although the catalytic sites of TR and hTrx are reversibly inhibited by the 2-imidazolyl disulfides, it is the irreversible thioalkylation of Cys73 of hTrx by the disulfides that most probably accounts for the inhibition of thioredoxin-dependent cell growth by the disulfides.


