TRAP-6PAR1 agonist CAS# 141136-83-6 |
- Thrombin Receptor Agonist Peptide
Catalog No.:BCC3950
CAS No.:137339-65-2
- SLIGRL-NH2
Catalog No.:BCC3947
CAS No.:171436-38-7
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- AY-NH2
Catalog No.:BCC3949
CAS No.:352017-71-1
- ML161
Catalog No.:BCC3642
CAS No.:423735-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 141136-83-6 | SDF | Download SDF |
| PubChem ID | 9831933 | Appearance | Powder |
| Formula | C34H56N10O9 | M.Wt | 748.88 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Thrombin receptor activator peptide 6 | ||
| Solubility | H2O : 33.33 mg/mL (44.51 mM; Need ultrasonic) DMSO : ≥ 28 mg/mL (37.39 mM) *"≥" means soluble, but saturation unknown. | ||
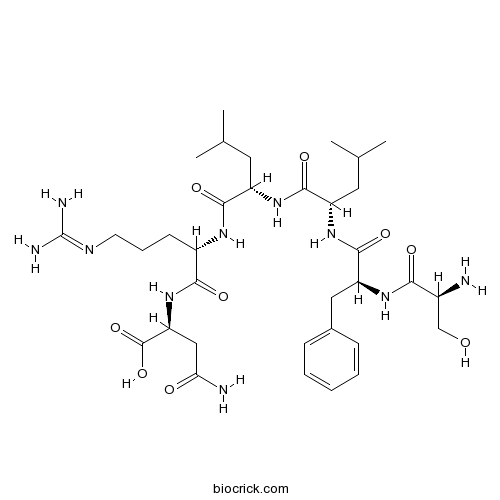
| Sequence | SFLLRN | ||
| Chemical Name | (2S)-4-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-hydroxypropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-4-oxobutanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC(CC(=O)N)C(=O)O)NC(=O)C(CC1=CC=CC=C1)NC(=O)C(CO)N | ||
| Standard InChIKey | HAGOWCONESKMDW-FRSCJGFNSA-N | ||
| Standard InChI | InChI=1S/C34H56N10O9/c1-18(2)13-23(30(49)40-22(11-8-12-39-34(37)38)29(48)44-26(33(52)53)16-27(36)46)42-31(50)24(14-19(3)4)43-32(51)25(41-28(47)21(35)17-45)15-20-9-6-5-7-10-20/h5-7,9-10,18-19,21-26,45H,8,11-17,35H2,1-4H3,(H2,36,46)(H,40,49)(H,41,47)(H,42,50)(H,43,51)(H,44,48)(H,52,53)(H4,37,38,39)/t21-,22-,23-,24-,25-,26-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Peptide fragment (residues 42-47) of protease-activated receptor 1 (PAR1) that acts as a PAR1 agonist. Stimulates platelet aggregation (EC50 = 0.8 μM), promotes intracellular Ca2+ mobilization and induces rapid phosphodiesterase 3A (PDE3A) phosphorylation in vitro. |

TRAP-6 Dilution Calculator

TRAP-6 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Peptide fragment (residues 42-47) of protease-activated receptor 1 (PAR1) that acts as a PAR1 agonist. Stimulates platelet aggregation (EC50 = 0.8 μM), promotes intracellular Ca2+ mobilization and induces rapid phosphodiesterase 3A (PDE3A) phosphorylation
- 12-Hydroxydodecanoic Acid
Catalog No.:BCN8405
CAS No.:505-95-3
- JNK-IN-8
Catalog No.:BCC1673
CAS No.:1410880-22-6
- Xanomeline oxalate
Catalog No.:BCC4146
CAS No.:141064-23-5
- 1-Hydroxy-2,3,4,7-tetramethoxyxanthone
Catalog No.:BCN6506
CAS No.:14103-09-4
- PACOCF3
Catalog No.:BCC7074
CAS No.:141022-99-3
- Gama-Tocotrienol
Catalog No.:BCN3720
CAS No.:14101-61-2
- Frangulin B
Catalog No.:BCC8175
CAS No.:14101-04-3
- 2-Thiouracil
Catalog No.:BCC4752
CAS No.:141-90-2
- Malonic acid
Catalog No.:BCN8534
CAS No.:141-82-2
- 2-Aminoethanol
Catalog No.:BCN1756
CAS No.:141-43-5
- Ricinoleic acid
Catalog No.:BCC8248
CAS No.:141-22-0
- Nerylacetate
Catalog No.:BCN3802
CAS No.:141-12-8
- Epiguajadial B
Catalog No.:BCN4075
CAS No.:1411629-26-9
- 2-(Dimethylaminomethyl)-2-propanol
Catalog No.:BCN1774
CAS No.:14123-48-9
- Btk inhibitor 1
Catalog No.:BCC4238
CAS No.:1412418-47-3
- Asenapine hydrochloride
Catalog No.:BCC1371
CAS No.:1412458-61-7
- QS-21
Catalog No.:BCC8243
CAS No.:141256-04-4
- PEPA
Catalog No.:BCC5951
CAS No.:141286-78-4
- Orobanchyl acetate
Catalog No.:BCN7779
CAS No.:1413843-71-6
- Trachelanthamine
Catalog No.:BCN2041
CAS No.:14140-18-2
- PX 12
Catalog No.:BCC2436
CAS No.:141400-58-0
- MRS 2219
Catalog No.:BCC6966
CAS No.:14141-47-0
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- Diosgenin glucoside
Catalog No.:BCN1250
CAS No.:14144-06-0
Is TRAP-6 suitable as a positive control for platelet reactivity when assessing response to clopidogrel?[Pubmed:20624009]
Platelets. 2010;21(7):515-21.
Adenosine 5'-diphosphate (ADP) inducible aggregation is used to assess platelet response to thienopyridines. Thrombin receptor-activating peptide-6 (TRAP-6) inducible aggregation may serve as a positive control because it acts via the thrombin receptor protease-activating receptor-1, which is not blocked by thienopyridines. We therefore investigated if TRAP-6 is suitable as a positive control when assessing residual platelet reactivity to ADP. Platelet response to clopidogrel was assessed in 200 patients on dual antiplatelet therapy using ADP inducible platelet aggregation by light transmission aggregometry (LTA), multiple electrode aggregometry (MEA), and the shear-dependent Impact-R. Test specificities were monitored by TRAP-6 inducible platelet aggregation. The aggregation-independent vasodilator-stimulated phosphoprotein (VASP) phosphorylation assay served for comparisons. ADP inducible aggregation was correlated to that by TRAP-6 (r = 0.33 to 0.72; p < 0.001 for all assays). A linear correlation was seen within MEA (r = 0.72). LTA TRAP-6 correlated weakly with the VASP assay (r = 0.19; p = 0.01), while there were no correlations of TRAP-6 responses by MEA or the Impact-R with the VASP assay (r = 0.03 and −0.09; p > 0.05). In all three assays, differences between ADP and TRAP-6 inducible aggregation varied considerably. Within MEA, TRAP-6 inducible aggregation was almost always stronger than ADP inducible aggregation, while within LTA and the Impact-R, weak responses to ADP were associated with both, weak and strong responses to TRAP-6. In conclusion, the application of TRAP-6 as a positive control for platelet reactivity has major limitations and results need to be cautiously interpreted on an individual basis.
Effect of halothane and isoflurane on binding of ADP- and TRAP-6- activated platelets to leukocytes in Whole blood.[Pubmed:11753011]
Anesthesiology. 2002 Jan;96(1):117-24.
BACKGROUND: Adhesion of activated platelets to neutrophils and monocytes has an important role in the regulation of inflammatory processes. This study investigates whether halothane and isoflurane affect binding of activated platelets to leukocytes in human whole blood. METHODS: Citrated whole blood was incubated for 60 min with either 1 or 2 minimum alveolar concentration (MAC) halothane or isoflurane. After stimulation with adenosine-5-diphosphate (ADP) or the thrombin receptor agonist protein TRAP-6, platelet-leukocyte adhesion and surface expression of CD62P on platelets were evaluated by flow cytometry. RESULTS: Halothane led to an inhibition of agonist-induced adhesion of activated platelets to neutrophils and monocytes. One MAC halothane reduced the formation of TRAP-6-induced platelet-monocyte conjugates. After exposure to 2 MAC halothane, agonist-induced platelet-monocyte and platelet-neutrophil adhesion were inhibited. Surface expression of CD62P on ADP- and TRAP-6-stimulated platelets were significantly reduced after 1 and 2 MAC halothane. After 2 MAC isoflurane, the authors observed an increase of the percentage of lymphocytes with bound platelets after activation with ADP. The percentage of neutrophils with bound platelets after activation with ADP or TRAP-6 was also increased in this group. Two MAC isoflurane led to an increase of the percentage of platelets expressing CD62P in the unstimulated and TRAP-6 stimulated samples, and of the amount of CD62P epitopes on the surface of platelets in the ADP-stimulated samples. CONCLUSION: This study indicates that halothane inhibits, whereas isoflurane enhances, adhesion of agonist-activated platelets to leukocytes. Interaction of both anesthetics with the expression of CD62P on platelets contribute to theses effects.
Enhanced platelet aggregation with TRAP-6 and collagen in platelet aggregometry in patients with venous thromboembolism.[Pubmed:12565719]
Thromb Res. 2002 Sep 15;107(6):325-8.
The role of platelet hyperaggregability as a possible risk factor for venous thromboembolism is not well defined. Some authors described enhanced maximal platelet aggregation in platelet aggregometry as a contributing factor for arterial and venous thrombosis. This syndrome has been termed "sticky-platelet syndrome" (SPS). The diagnosis of SPS is based on the demonstration of platelet hyperaggregability in aggregometry after stimulation with epinephrine (EPI) and/or adenosine diphosphate (ADP). We investigated platelet hyperaggregability in platelet-rich plasma (PRP) of patients (n = 34) with unexplained venous thromboembolism in comparison to healthy individuals (n = 53). For analysis, platelet aggregometry was performed and the influence of epinephrine, adenosine diphosphate, collagen (Coll) and thrombin receptor-activated peptide (TRAP-6) as agonist were determined. Compared to the control group, patients with venous thromboembolism showed an enhanced maximal platelet aggregation with low concentrations of TRAP-6 (2 microM) and collagen (0.05 microM). In contrast, we could not detect an increased platelet aggregation with EPI or ADP. Our results indicate that platelet hyperaggregability may represent an independent risk factor in patients with otherwise unexplained venous thromboembolism. In our study, low concentrations of TRAP-6 and collagen are superior to EPI and ADP to define platelet hyperreactivity in platelet aggregometry.
Protein kinase C-mediated phosphorylation and activation of PDE3A regulate cAMP levels in human platelets.[Pubmed:19261611]
J Biol Chem. 2009 May 1;284(18):12339-48.
The elevation of [cAMP](i) is an important mechanism of platelet inhibition and is regulated by the opposing activity of adenylyl cyclase and phosphodiesterase (PDE). In this study, we demonstrate that a variety of platelet agonists, including thrombin, significantly enhance the activity of PDE3A in a phosphorylation-dependent manner. Stimulation of platelets with the PAR-1 agonist SFLLRN resulted in rapid and transient phosphorylation of PDE3A on Ser(312), Ser(428), Ser(438), Ser(465), and Ser(492), in parallel with the PKC (protein kinase C) substrate, pleckstrin. Furthermore, phosphorylation and activation of PDE3A required the activation of PKC, but not of PI3K/PKB, mTOR/p70S6K, or ERK/RSK. Activation of PKC by phorbol esters also resulted in phosphorylation of the same PDE3A sites in a PKC-dependent, PKB-independent manner. This was further supported by the finding that IGF-1, which strongly activates PI3K/PKB, but not PKC, did not regulate PDE3A. Platelet activation also led to a PKC-dependent association between PDE3A and 14-3-3 proteins. In contrast, cAMP-elevating agents such as PGE(1) and forskolin-induced phosphorylation of Ser(312) and increased PDE3A activity, but did not stimulate 14-3-3 binding. Finally, complete antagonism of PGE(1)-evoked cAMP accumulation by thrombin required both G(i) and PKC activation. Together, these results demonstrate that platelet activation stimulates PKC-dependent phosphorylation of PDE3A on Ser(312), Ser(428), Ser(438), Ser(465), and Ser(492) leading to a subsequent increase in cAMP hydrolysis and 14-3-3 binding.
PAR 1-type thrombin receptors are involved in thrombin-induced calcium signaling in human meningioma cells.[Pubmed:10421070]
J Neurooncol. 1999 Apr;42(2):131-6.
Thrombin is known to play a role as regulator in tumor spreading and tumor growth. Proteinase-activated receptor 1 (PAR 1)-type thrombin receptors were identified in different cancer cells including human glioblastoma cells. Thus a function of PAR 1 in brain tumors may be suggested. In this study, the presence of PAR 1-type thrombin receptors was investigated in primary cell cultures established from operated human meningiomas from two 59- and 79-year-old women. Characterization of PAR 1 on binding level was performed using immunofluorescence studies with the monoclonal anti-PAR 1 antibody Mab 61-1 directed against a domain in the NH2-terminus of PAR 1. These binding sites constitute functional thrombin receptors that are involved in thrombin-induced signaling in human meningioma cells as demonstrated by investigation of alpha-thrombin- and PAR 1-activating hexapeptide (TRAP-6)-induced [Ca2+]i mobilization. To our knowledge, this is the first report demonstrating thrombin-induced intracellular signaling in human meningioma cells mediated by the PAR 1-type thrombin receptor.
Structure-function relationships in the activation of platelet thrombin receptors by receptor-derived peptides.[Pubmed:1313429]
J Biol Chem. 1992 Mar 25;267(9):6081-5.
According to present models, thrombin activates platelets by cleaving its receptors after Arg41, creating a new N terminus which acts as a tethered ligand. In support of this model, a peptide (SFLLRNPNDKYEPF or TRP42/55) corresponding to residues 42-55 has been shown to activate the receptor. In the present studies, the structural basis for thrombin receptor activation was examined using fragments of this peptide, as well as variants of the peptide with selected amino acid substitutions. The results show that the features of SFLLRNPNDKYEPF required to mimic the effects of thrombin reside within the first 6 residues, SFLLRN. A hexapeptide comprised of these residues was approximately 5 times more potent than the parent peptide in assays of platelet aggregation and, in addition, caused tyrosine phosphorylation, inhibition of cAMP formation, and an increase in cytosolic Ca2+. Omission of either the Ser residue or the Arg and Asn residues greatly diminished peptide activity, as did the substitution of Ala for Phe or Arg. Substitution of Ala for Ser or the initial Leu, on the other hand, had little adverse effect. The inactive peptides SALLRN and NPNDKYEPF had no effect on platelet activation initiated by SFLLRN, but FLLRN inhibited platelet aggregation in response to both SFLLRN and thrombin. These results suggest that within SFLLRN the Phe and Arg residues are particularly important and that Phe must be preceded by another amino acid, the identity of which is not tightly constrained. This observation and comparisons with the homologous domains of proteins whose tertiary structure is known were used to predict the conformation of the SFLLR sequence. The model which emerged suggests that the SFLLR domain may be part of an extended beta structure in the intact receptor and that cleavage by thrombin causes it to contract and assume a modified helical configuration. In this predicted conformation the side chains of Phe and Arg point in the same direction, potentially into a pocket formed by the remainder of the receptor.


